Functional Group Name
|
Functional Group
|
General Formula
|
Comments
|
Alcohol
|
-OH
|
ROH
|
R is an abbreviation
for a hydrocarbon group like methyl,CH3-,
or ethyl, CH3CH2- or something more complex.
Just remember an R group has a carbon atom bonded to the
oxygen atom. (Go
here for more help on alcohol and ether functional
groups.)
|
ether
|
-O-
|
ROR
|
Ether have an oxygen atom which both bonds are to
carbon atoms. (Go
here for more help on alcohol and ether functional
groups.)
|
Carboxylic acid
|
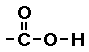
|
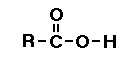
|
In carboxylic acids one of the oxygens has a
double bond the other oxygen atom is bonded to a
hydrogen. (Go
here for more help on carboxylic acid functional
group.)
|
Ester
|
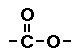
|
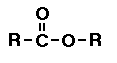
|
In esters one of the oxygens has a double bond
the other oxygen atom is bonded to a carbon atom of a
hydrocarbon. (Go
here for more help on the ester functional group.)
|
Amine
|
N
|
RNH2,
R2NH, R3N
|
In amines a nitrogen is bonded to one, two or
three carbon atoms. Amines are a derivative of ammonia,
NH3 where hydrogen atoms
are succesively replaced with R- groups. (Go
here for more help on amine functional group.)
|
Aromatic
|
|
CnHn
|
Aromatic compounds have rings of carbon atoms
with alternating double and single bonds. (Go
here for more help on aromatic functional group.)
|