Amine Functional Group
If you would like to learn about the amine functional groups
with some questions go here instead of
continuing with this page.
Amines are organic compounds (hydrocarbons) which contain the
element nitrogen. Everyone knows ammonia a compound of nitrogen
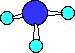
and hydrogen with the formula NH3. The structure of
ammonia is shown in several different forms below,
Ball-and-Stick
|
Lewis Structure
|
Electron-dot Structure
|
|
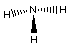
|
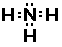
|
Notice there are three bonds to the nitrogen in this compound
and a lone-pair of electrons on the nitrogen. We can only see the
lone-pair in the right most Electron-dot structure. It is present
in the other two structures, it just is not shown.
Amines can be thought of as derivatives of ammonia. Amines
contain at least one carbon containing substituent bonded to the
nitrogen in place of one of the hydrogen atoms. So if we replace
one hydrogen atom with the simplest carbon containing branching
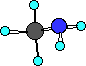
group we would have the following compound, CH3NH2.
what does this compound look like?
Ball-and-Stick
|
Lewis Structure
|
Electron-dot Structure
|
|
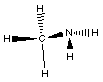
|
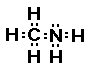
|
This compound is called methyl amine and is the simplest
amine. It is no longer ammonia because we have replaced one of
the hydrogens with a carbon containing substituent.
Another way to look at these compounds is as an alkane with a
nitrogen atom replacing one of the hydrogen atoms.
The important thing to remember is just as all organic
compounds have carbon atoms with four bonds, all amine compounds
will have nitrogen with three bonds. In methyl amine the nitrogen
has three bonds; two to hydrogen atoms and one to a carbon atom.
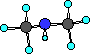
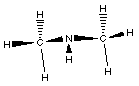
The next simple amine is dimethylamine. Just as the name
suggeests this amine has two methyl groups bonded to the
nitrogen.
Ball-and-Stick
|
Lewis Structure
|
Electron-dot Structure
|
|
|
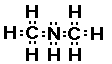
|
This compound has a nitrogen atom with three bonds; two to
carbon atoms and one to a hydrogen atom.
We could continue with trimethylamine, or ethylamine as
additional examples of amines. But lets look at some more
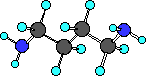
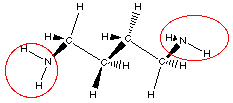
disgusting examples of amines!! Have you ever smelled putrescine
or cadaverine? These amines are found in urine and bad breath.
How would you know these are amines, well not from the names, but
by finding the functional group given the structure. Here are the
structures of these two very smelly compounds.
Compound
|
Ball-and-Stick
|
Lewis Structure
|
Electron-dot Structure
|
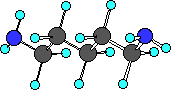
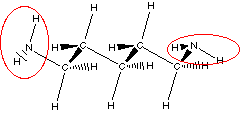
putrescine
|
|
|
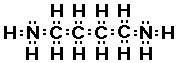
|
cadaverine
|
|
|
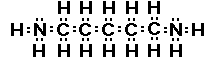
|
Do you see there are actually two amine groups in putrescine
and in cadaverine.
So we can recognize amine functional group by locating a
nitrogen atom with three bonds one of which is to a carbon atom.
We can use the notation R- as a carbon containing substituent. So
the amine functional group is RNH2, or R2NH,
or R3N.
Return
to man functional group page.