Aromatic Functional Group
The aromatic hydrocarbons are a fourth class of organic
compounds with only carbon and hydrogen. We recognize the
alkanes, alkenes and the alkynes from their formulas which follow
the general formula as shown below,
Hydrocarbon
|
Formula
|
alkanes
|
CnH2n+2
|
alkenes
|
CnH2n
|
alkynes
|
CnH2n-2
|
The aromatics are a fourth group,
Hydrocarbon
|
Formula
|
aromatic
|
CnHn
|
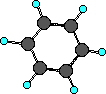
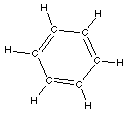
The only member of the aromatic group we will spend time on
is benzene, C6H6. It looks like,
Functional Group
|
Ball-and-stick
|
Lewis Structure
|
aromatic (benzene)
|
|
|
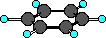
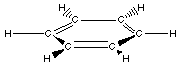
Recognizing the aromatic group consists of finding the
six-membered ring of carbon atoms. Although the ball-and-stick
model above does not clearly show it the benzene ring is planar.
Functional Group
|
Ball-and-stick
|
Lewis Structure
|
aromatic (benzene)
|
|
|
Benzene is a liquid at room temperature and is an important
solvent because organic compounds are more soluble in it than in
water.
Lets look at some compounds that contain the aromatic benzene
(benzyl) group.
Compound
|
Ball-and-Stick
|
Lewis Structure
|
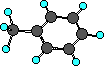
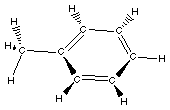
Toluene (methyl benzene)
|
|
|
Phenol
|
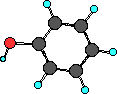
|
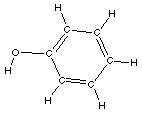
|
Styrene
|
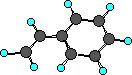
|
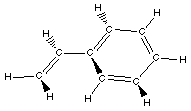
|
Phenol is interesting as it has an alcohol functional group
as well as the aromatic functional group.
Return
to man functional group page.