- 1. Using a vacuum pump, evacuate a bell jar containing a small quantity
of shaving cream or a marshmallow. Expansion rules!
2. Obtain a small vial (without cap). Put a bit of shaving cream in
vial mouth to “plug” the opening. Place vial in the bell jar. Evacuate
the bell jar and watch the shaving cream expand.
1. Pictures in the mind of gas filling a room.Pressure = Force/Area
2. Pictures and discussion of formation of ammonium chloride, NH 4 Cl:
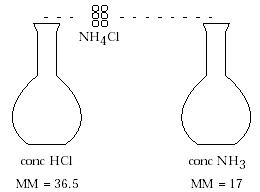
HCl(g) + NH3(g) ---> NH4 Cl(s)
Gas Gas White solidThe solid is formed closer to the HCl source because the less dense gas (NH3 ) diffuses faster.
1. Difference between spike heel and large heel . Because spike heel has smaller area, it will exert more pressure on the floor than large heel, assuming the force (body weight) is the same.Temperature2. Atmospheric pressure (effect on body, changes on mountain). Compare to changes of state. Atmospheric pressure decreases as you move away from the earth because there are fewer gas molecules per unit volume. At high altitudes, we may have trouble breathing because of a lower O2 concentration and because of the reduced pressure. The reduced pressure also affects the change from liquid to gas; the boiling point is lowered because the atmospheric pressure is lower. (Boiling point is the temperature at which the vapor pressure equals atmospheric pressure). Cake mixes will have different directions for higher altitudes. (Show cake mix box.) It also takes longer for boiled foods to cook at high altitudes due to the lowered boiling point.
3. Design and use of a barometer (construction of barometer involves safety hazards). Convert barometric pressure in inches of mercury to cm Hg, to mmHg.
1 inch = 2.54 cm.4. Measure blood pressure
5. Question. How tall would a barometer column under one atmosphere pressure need to be if the column were filled with water, not mercury?
[13.53 x 760 mmHg = the ratio of density of Hg to that of water]
1. Discussion of Kelvin scale in relation to pressure and volume .Pressure vs. Temperature
Temperature on Neptune –450 °F. Calculate in kelvins. Does this prove or disprove the concept of absolute zero?2. Relate temperature to kinetic energy . Temperature is related to the average kinetic energy (motion) of molecules. Kelvin temperature is proportional to the kinetic energy.
3. Discuss difference between heat and temperature. Draw pictures of filled tea cup and filled bathtub. If at the same temperature, which contains more heat?
1. Auto tires in summer and winter. You may need to let air out to decrease pressure in hot weather. Add air in cold weather.Volume vs. Temperature2. Why does a bicycle pump get hot when you use it?
1. Hot-air balloons. What happens if the hot-air balloon heater malfunctions?Pressure vs. Volume
If the air cools, its density will increase and the balloon gas will no longer be “lighter” (less dense) than air.2. When gases are produced suddenly with the accompanying release of energy, explosions occur. This is how gun powder propels bullets, and it explains the force produced by rocket fuel. A typical rocket reaction:
2N2H4(l) + N2O4(g) ---> 4H2O(g) + 3N2(g) (Hydrazine) (Nitrogen
tetroxide)
Notice the relatively large number of moles of gas produced.
Syringe: As you push in plunger of a sealed syringe (increasing pressure), the volume decreases.
Amount of Gas vs. Volume
1. Blow up auto or bicycle tire, blow up balloon. This adds more moles of gas (n increases); the volume increases.Amount (Moles) vs. Pressure2. Avogadro’s Law. Avogadro’s law states that equal volumes of gases at the same temperature and pressure contain the same amount of gas. One mole of any gas at standard temperature and pressure (1 atm and 0 °C) occupies 22.4 L. Use general gas law (PV = nRT) to calculate this value.
1. Pressure is used by anesthesiologists to measure amount of gases. When you buy gases such as helium in cylinders of fixed volume, the amount of gas can be calculated from the pressure (assuming temperature and tank volume are known). Gases are very difficult to weigh; pressure is easy to measure, so it is simpler to use pressure to determine the amount of gas.Gas Densities2. When you add air to an auto tire, you increase the gas pressure in the tire.
Balloons, dirigibles, and blimps float in air because they contain gases that are less dense (lighter) than ordinary air. Helium, hydrogen, or hot air may be used. The densities of these gases are all less than the surrounding air, since the mass of gas in a given volume is less. When unconfined air is heated, its molecules move faster (on average) and the volume expands. Hydrogen and helium have lower molar masses than most gases composing air (such as oxygen, nitrogen, water vapor, carbon dioxide, etc.). Dirigibles were used often in World Wars I and II for observation and transportation. Germany used hydrogen gas because it had no access to helium, which is found in large quantities in the U.S. Hydrogen is very explosive; the German airship Hindenberg exploded near Lakehurst, New Jersey in 1937, with great loss of life.Mixtures of Gases and the Atmosphere
The atmosphere is a mixture of 80% nitrogen and 20% oxygen with traces of argon and other gases. Total atmospheric pressure is the sum of pressures of each individual gas. Even though oxygen and nitrogen have different densities, they mix completely in the atmosphere because of the ability of a gas to fill any space it occupies.