Amino acids can combine easily by removing a water molecule, joining the amino end
of one amino acid with the carboxyl end of another. The resulting bond is called a
peptide bond. The process of peptide bond formation is identical to the process of
condensation polymerization forming nylon (see Polymers module).For example, the
amino acids glycine and alanine can combine to form a dipeptide, glycylalanine:
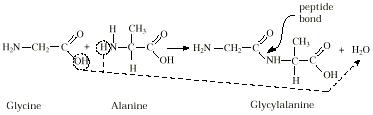
When several amino acids are joined in such a manner, the resulting structure is
called a peptide. Examining the way the peptide bond is formed, it is obvious that
it could form by connecting the amino and carboxyl end of either amino acid. Thus,
there could be two different ways to join glycine and alanine. For example,

Although glycylalanine and alanylglycine, contain the same two amino acids, they
are two completely different compounds. In fact, if 4 different amino acids are
combined, and if each amino acid can appear more than one time in this sequence of
four, it would be possible to form 256 different compounds. How many different
compounds can be formed from all 20 amino acids? A quick calculation shows that
1026 compounds are possible! This mind-boggling number clearly shows the possibility
of billions of different proteins, all consisting of only 20 amino acids! The order in
which these amino acids are strung together is the protein’s primary structure.When
proteins are digested the process is reversed and peptide bonds are broken to release
amino acids. Since water is required, the process is called hydrolysis.As polypeptides get larger, cross-linking occurs. An important cross-linking bond is the
disulfide bond, which is formed by thiols (–SH groups). Thiols can react with heavy
metals, such as mercury and lead, which results in the alteration of protein structure.
This structural alteration accounts for heavy metal toxicity. As the protein molecule
becomes more complex, other bonds—ionic, hydrogen, and hydrophobic/hydrophilic—
become important in holding the giant molecule together. We can effectively break
these bonds and denature or destroy the protein using such things as heat, acids and
bases, alcohol, and detergents. Perhaps you can think of some practical examples of
protein denaturation (e.g., frying or whipping egg white).
TABLE OF CONTENTS TOPIC OVERVIEW CONCEPT/SKILLS DEVELOPMENT LINKS/CONNECTIONS EXTENSIONS