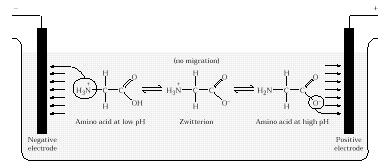
 When the amphoteric amino acid is placed in an electric field, it tends to migrate toAmino acids also have a unique property that
When the amphoteric amino acid is placed in an electric field, it tends to migrate toAmino acids also have a unique property that
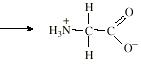
allows separation. They have both acid and base
properties. Such structures are said to be
amphoteric, and the double-ion produced is called
a zwitterion.
either the positive or negative pole, depending on the strength of its positive or
negative charge. However, if the pH of the solution containing the amino acid is
adjusted to the point that the true zwitterion exists, the amino acid has no net charge
and it will not migrate in an electric field. This point is called the isoelectric point for
the amino acid, and it is a characteristic property of each individual amino acidó
much like a fingerprint is characteristic of each individual. The procedure just
described is called electrophoresis, and provides a simple way to identify proteins
(see Forensic Chemistry module).
Scientists now know a great deal about proteins, and are constantly learning more
about these complex and fascinating macromolecules.Language of Chemistry
Proteins
a-helix model of protein structure in which intrachain hydrogen bonding holds
polypeptide coils together in a helix.amino acid compound containing both an amino group and a carboxylic acid
group in the same molecule. Amino acids are the building blocks of protein.amphoteric describing a substance that can act as either an acid or a base.
denatured protein protein that does not exist in the native state, but has been
altered.disulfide bonds bonds formed between sulfur atoms of polypeptide chains.
electrophoresis technique of regulating the migration of amino acids in an
electric field through pH manipulation.isoelectric point specific pH at which an amino acid has no net charge and
exists as a zwitterion.
TABLE OF CONTENTS TOPIC OVERVIEW CONCEPT/SKILLS DEVELOPMENT LINKS/CONNECTIONS EXTENSIONS