2. The Halogens
3. Word Search
4. Halogens Crossword Puzzle
Module developed by Ronald D. Archer, William G. Cumming, and Abraham
M.
Rennert, the New England
team.
Epstein, I. (1987, March 30). Patterns in time and space. Chemical
and Engineering
News, 65(13),
24-36.
A number of these oscillating reactions contain halogen compounds, but detailsFortman, J. J. (1993, September). A simpler tomato juice demonstration of halogen
may be beyond typical high school students. On the other hand, exceptionally
able students can find these challenging.
Franz, H. W., and Malm, E. (1968). Fundamental experiments for college
chemistry
(2nd Ed.). San Francisco,
CA: Freeman.
Greenwood, N. N., and Earnshaw, A. (1984). Chemistry of the elements. Oxford: Pergamon.
Hileman, B. (1988, August 1). Fluoridation of water. Chemical and
Engineering News,
66(31), 26-42.
Excellent information on the use of fluoride as a preventive measure for toothKolb, D. (Ed.). (1988). Journal of Chemical Education, 65(11), 1004-1005.
decay.
This short note contains two overhead projector oscillating-reactionMohrig, J. R., and Child, W. C., Jr. (1987). Chemistry in perspective . Newton, MA:
demonstrations appropriate for this module.
Good background source for this module.The New Encyclopaedia Britannica. (1990). Chicago, IL: Encyclopedia Britannica.
See Organic halogen compounds, Halogens, Photography (Technology of), inPryde, L. (1973). Environmental chemistry. Menlo Park, CA: Benjamin/Cummings.
this or other modern encyclopedia for additional information concerning
halogens.
Good background source for this module.Shakhashiri, B. Z. (1983). Chemical demonstrations: A handbook for teachers of
The following are relevant to this module: Demonstration 1.25: Reaction ofTopics in Chemistry: Ozone–Chlorofluorocarbons and the Hole in the Ozone Layer.,
Sodium and Chlorine; Demonstration 1.26: Reaction of Antimony and Chlorine;
Demonstration 1.28: Reaction of Aluminum and Bromine; Demonstration 1.29:
Reaction of White Phosphorus and Chlorine; and Demonstration 1.30: Reaction
of Red Phosphorus and Bromine.
Appendix
Transparency Masters
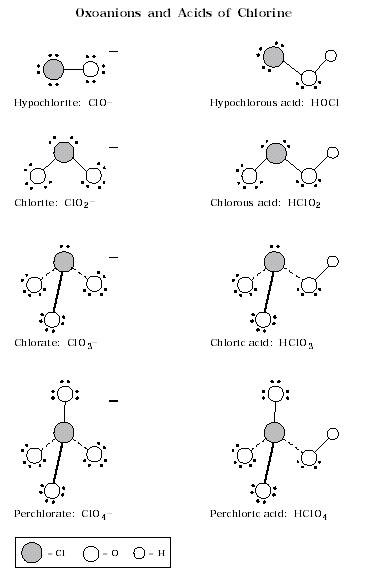
1. Oxoanions and Acids of Chlorine
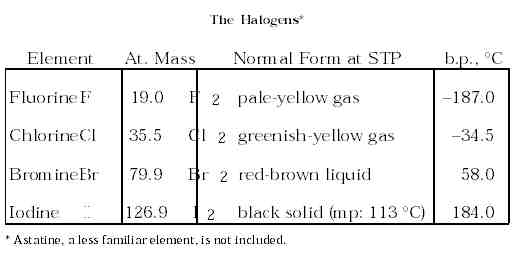
2. The Halogens
3. Word Search
4. Halogens Crossword Puzzle
| Table of Contents | Topic Overview | Concept (Lab 1) | Concept (Lab 2) | Demonstrations |
|
|
Humor |
Media | Links
Connections |
References
Appendix |
|---|