
6.3. Apply Dalton’s Law of partial pressure to calculate
the pressure of combined gases and to calculate the partial pressures of gases
in mixtures.
Each gas in a mixture exerts the same pressure they would exert if they were
in the container alone.
For example, if a mixture of H2, N2, and Br2
gases at a given temperature, T, and a volume, V exert a total pressure that
can be expressed in terms of the partial pressure of each of the gases.
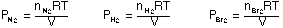
The total pressure can be expressed as
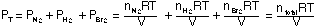
Proceed to the following tutorial (PT#5) to
apply Dalton's Law of Partial Pressure. Problem
Tutorial
1 2 3 4
next



![]()
