
6.3. Apply Dalton’s Law of partial pressure to calculate
the pressure of combined gases and to calculate the partial pressures of gases
in mixtures.
Dalton's law of partial pressures states that the total
pressure exerted by a mixture of gases is the sum of partial pressure of each
individual gas present. Each gas is assumed to be an ideal gas.

Where P1, P2, etc. are the partial
pressure of gas 1 and gas 2 in the mixture.
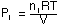
Since each gas behaves independently, the ideal gas law can be used to calculate
the pressure of that gas if we know the number of moles of the gas, the total
volume of the container, and the temperature of the gas.
1 2 3
4 next



![]()
