 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Molecular Geometry
Chapter 9
Objectives
Following your study of this chapter, you should be able to
![]()
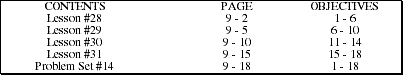
![]()
![]()

1a. Sketch a five-atom molecule that has a central atom to which the remaining four atoms are independently attached. All bond angles are equal and all bond lengths are equal. In your sketch label bond angels and bond lengths.
![]()
![]()
2a. Draw the Lewis electron dot structure for CCl4 and identify all the bonding pairs
and nonbonding pairs of electrons.
![]()
![]()

3. Differentiate between electron-pair geometry and molecular geometry.
![]()
4. List the steps used to predict the molecular geometry using VSEPR.
![]()
![]()
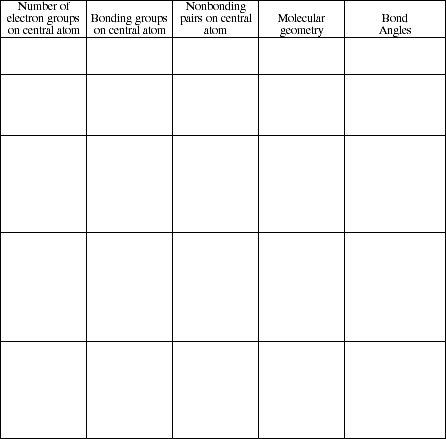
5. Complete the following table. Note: Be sure to include all possible combinations of bonding and nonbonding electrons on the central atom.)
![]()
![]()

![]()
6. How does the change in the number of bonding electrons and nonbonding
electrons affect the molecular geometry for the series AB4, AB3N, AB2N2.
(Note: A is the symbol for the central atom, B is the symbol for a bonding group
of electrons and N is the symbol for a nonbonding pair of electrons.)
![]()
7. In which molecular geometry is it necessary to differentiate between axial and
equatorial atoms?
![]()
![]()

![]()
![]()
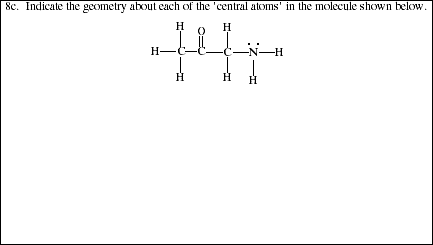
8. How does predicting molecular geometries differ for molecules with multiple 'central atoms' compared to molecules with a single central atom?
![]()

![]()
![]()
![]()

![]()
![]()
10a. Define the term dipole moment.
![]()

![]()
![]()

11a. Define the term overlap as it relates to interaction of atomic orbitals on atoms.
![]()

![]()
![]()

![]()
![]()
12. Explain the difference between the model for a sigma (s) bond and for a pi (p) bond. Clarify the distinction between the overlaps by drawing a picture of two 'p' orbitals forming a sigma (s) bond and two 'p' orbitals forming a pi (p) bond.
![]()
![]()
13. Given four atomic orbitals, an s orbital and three p orbitals and the resulting set of four sp3 hybrid orbitals, explain the difference in shape, orientation, size and
![]()
![]()
14. Complete the following table by filling in the missing information.
![]()
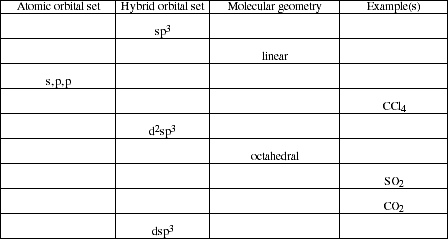
![]()
![]()

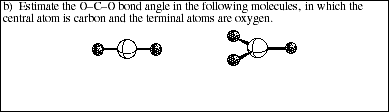
15. Which hybrid orbitals are used to describe multiple bonding between carbon and oxygen in the following molecules?
![]()

![]()
16. Explaining the meaning of the term delocalized as it applies to binding electrons
and how the term is used to explain the structure of benzene, C6H6.
![]()
![]()
17. Describe some of the physical properties (phase, color, melting point, boiling point) of oxygen, ozone, sulfur, carbon and silicon.
![]()
![]()
18. Draw the Lewis structure of each of the elemental forms of oxygen, ozone, sulfur, carbon (simple sketch of graphite or diamond) and silicon.
![]()
![]()
Problem Set #14
AP Chemistry by Satellite
ALL work must be shown in all problems for full credit.
PS14.1. Complete the following table
![]()
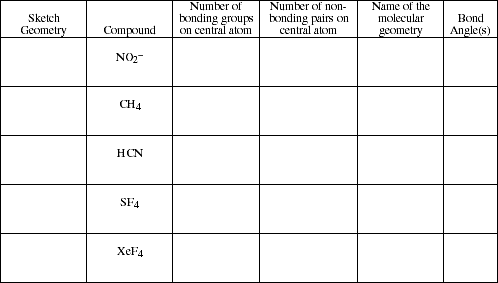
PS14.2. Use simple structure and bonding models to account for each of the following.
![]()
a). The H-N-H bond angle is 107.5 º in NH3.
![]()
b). The I3- ion is linear.
![]()
![]()
PS14.3. Which of the molecules listed in Problem #14.1 above are polar and which are nonpolar?
![]()
PS14.4. Consider theLewis structure for glycine, the simplest amino acid:
![]()
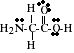
![]()
a). What are the approximate bond angles about each of the two carbon atoms,
and what are the hybredizations of the orbitals on each of them?
![]()
b). What are the hybridizations of the orbitals on the two oxygens and the
nitrogen atom, and what are the approximate bond angles about the nitrogen?
![]()
c). What is the total number of s bonds in the entire molecule? Sigma (s) bonds?
![]()
PS14.5. Indicate the hybridzation on the central atom for each of molecules in Problem
14.1 above.
![]()
PS14.6. Indicate the atomic orbitals on each atom in the following molecules which are
involved in forming the covalent bond.
a). H-F
![]()
b). F2
![]()
c). ![]()
![]()
![]()
PS14.7a. Draw two resonance structures for the nitrite ion, NO2-.
![]()
b). What is the hybridization around the nitrogen atom in this polyatomic ion?
![]()
c). Describe the pi-bonding in the polyatomic ion.
![]()
d). Label both the sigma and the pi bonds as either localized or delocalized.
![]()
PS14.8. Use simple structure and bonding models to account for the following.
The bond lengths in CO32- are all identical and are shorter than a carbon-
oxygen single bond.
![]()
![]()
Microcomputer software
Project SERAPHIM
Dr. John Moore
Department of Chemistry
University of Wisconsin
Madison, WI 53706
AP401 ISOMERS
VSEPR
![]()
![]()
Computer Aided Instruction for General Chemistry by William Butler & Raymond Hough
Drill-and-practice software
$40 (4-disk set)
John Wiley & Sons, Inc.
605 3rd Avenue
New York, NY 10158
(this software may not be available)
Diskette #2 Molecular Bonding and Structure
![]()
![]()