 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Introduction to Quantum Theory
Chapter 6
Objectives
Following your study of this chapter, you should be able to
![]()
![]()
![]()

1a. Identify the color metals glow when heated.
![]()
b) Identify the color of the following substances and the color of the corresponding
metallic ion when placed in the flame of a Bunsen burner.
![]()
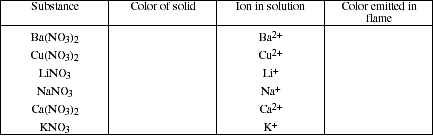
2. Define the terms:
electromagnetic radiation
![]()
wave
![]()
![]()
2. (Continued)
wavelength
![]()
frequency
![]()
amplitude
![]()
velocity
![]()
![]()
3a. Write the equation that describes the mathematical relationship between
wavelength and frequency using the standard symbol for each quantity.
![]()
![]()
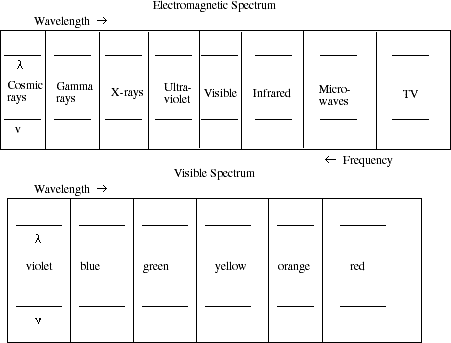
4. Identify the frequencies and wavelengths associated with the various regions of
the electromagnetic spectrum.
 [!] Frequency
[!] Frequency
![]()
![]()
5. Describe the difference between the appearances of an emission spectrum and an absorption spectrum for any element.
![]()
![]()

6. Define quantization. What is a quantum of matter? What is a quantum of light (radiant energy)?
![]()
7. Describe the experiment which demonstrates the photoelectric effect. How did
Einstein explain the photoelectric effect?
![]()
8a. Write the relationship between the energy of a photon of light and its frequency.
![]()
![]()

![]()
![]()
9a. List the postulates about the behavior in atoms that Bohr invoked to explain the
occurrence of emission lines and their associated energies in the hydrogen
spectrum.
![]()
b) Write the mathematical equation that yields experimentally observed values for
frequencies of light in the Balmer series, the Paschen series, the Bracket series
and the Pfund series.
![]()
![]()
10. Identify the principal quantum number in the mathematical equation that Bohr used to explain the hydrogen emission spectra series listed above.
![]()
11. Write the equation, based on the Bohr model, which determines the energy of an
electron in a particular orbit of the hydrogen atom.
![]()
![]()

![]()
![]()
Problem 11a &b(Continued)
![]()
![]()
11c. Use the energy diagram produced in #11a to illustrate a transition that results in emission of radiant energy. Does the energy of an electron increase or decrease when energy is emitted? Next illustrate a transition that results in the absorption of radiant energy.
![]()
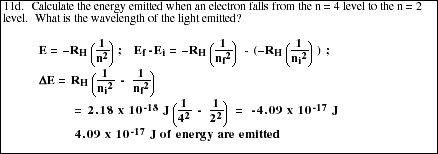
![]()
![]()
Problem Set #9
AP Chemistry by Satellite
ALL work must be shown in all problems for full credit.
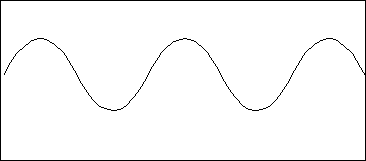
PS9.1. In the wave form shown below label the wavelength and amplitude. Describe how the frequency of the wave can be determined.
![]()
![]()
PS9.2. The double line in the sodium emission spectrum have wavelengths of 5.896 x
10-5 cm and 5.890 x 10-5 cm (589.6 and 589 nm). Calculate the frequency of
each line.
![]()
PS9.3. The wavelengths of the sodium doublet can be accurately measured despite the
fact that they are very short. The IUPAC standard for the meter is, in fact,
based on the wavelength of a particular line in the emission spectrum of the
element krypton. According to the standard, one meter is defined as
1,650,763.73 wavelengths. What color is this line?
![]()
![]()
PS9.4. Briefly describe the difference between the continuous model of matter and quantized view of matter.
![]()
PS9.5. Which color of light is higher energy, blue of red? Explain.
![]()
PS9.6. An argon laser emits light with a wavelength of 488 nm. Calculate the energy
of a photon of light emitted from the laser.
![]()
PS9.7. The wavelength of a photon of light capable of breaking a carbon-carbon single
bond is 344 nm. What area of the electromagnetic spectrum is light of this
wavelength located? Calculate the energy required to break 1 mol of C-C single
bonds.
![]()
![]()
PS9.8. Calculate the energy and the wavelength of light emitted when an electron in an excited hydrogen atom falls from the n = 5 level to the n = 1 level.
![]()
PS9.9. An electron initially in the n = 2 energy level in a hydrogen atom absorbs a
photon of light with a frequency of 6.167 x 1014 s-1. Calculate the new energy
level the electron will occupy.
![]()
PS9.10. Will a photon of light of wavelength 480 nm excite an electron in the hydrogen
atom from the n = 1 level to the n = 2 level? Explain.
![]()
![]()

12a. Define the terms excited state, ground state and ionization energy.
![]()

13. What was the importance of De Broglie's postulate of the wave nature of matter?
![]()
![]()
14. Define the uncertainty principle and explain its importance.
![]()
15. What are the critical shortcomings of the Bohr model of the atom? How does the
quantum mechanical description of the atom overcome these shortcomings.
![]()
16. Define the term orbital.
![]()
17a. Define the three quantum numbers, principal, azimuthal and magnetic using
mathematical relationships. How are these quantum numbers related to the terms
shell, subshell and orbital?
![]()
17b. How are these quantum numbers related to energy, shape and orientation of the
electron?
![]()
![]()

18a. Prepare a table of the possible sets of quantum numbers for an electron in the n = 1, 2, 3 and 4 levels.
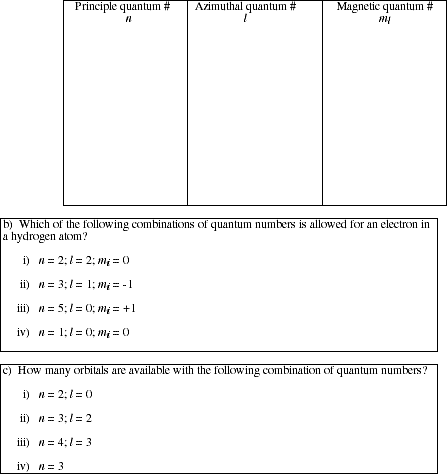
![]()
![]()
19a. Draw a detailed energy level diagram for the orbitals for the first four principal quantum levels. Label each set of orbitals with the correct shell and subshell designation, i.e., 1s, 2s, 2p, etc.
![]()
![]()
![]()
![]()
20. Draw the contour representation that depicts the shape of an s orbital, a px, py, pz orbital, and a dxz
![]()
21. How does the contour representation of a 1s orbital differ from that of a 2s
orbital?
![]()
![]()
Problem Set #10
AP Chemistry by Satellite
ALL work must be shown in all problems for full credit.
PS10.1. How do electrons shells, subshells and orbitals differ?
![]()
PS10.2. What are the possible values of l, and ml for the n = 2 shell?
![]()
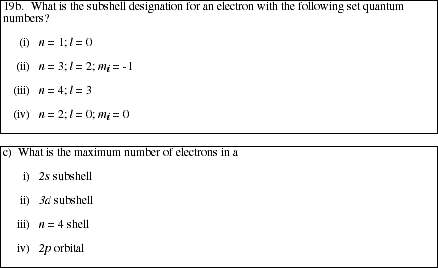
PS10.3. What are the set of quantum numbers for an electron in a 2s orbital? A 3d
sublevel? A 6f orbital?
![]()
PS10.4. Which of the following sets of quantum numbers describe an electron in an
excited state in a hydrogen atom? Which describe a ground state? Which are
not allowed?
![]()
a) n = 3; l = 3; mi = 0
b) n = 2; l = 1; mi = -1
c) n =1 ; l = 0; mi = 0
d) n = 2; l = 0; mi = +1
e) n = 5; l = 2; mi = +2
![]()
PS10.5. How many orbitals are available in each of the following subshells or shells?
![]()
a) n = 3 shell
b) 2p subshell
c) 4d subshell
d) n = 6 shell
![]()
![]()
PS10.6. Sketch a 1s subshell and a 2s subshell. What are the major similarities and differences between the two subshells.
![]()
![]()
Microcomputer software
![]()
Introduction to General Chemistry by Stan Smith, Ruth Chabay and Elizabeth Kean
Drill-and-practice software
$500 (10-disk set)
Falcon Software
P.O Box 200
Wentworth, NH 03282
1-603-764-5788
Diskette #3 Chemical Formulas and Equations
Diskette #6 Chemaze
![]()
Computer Aided Instruction for General Chemistry by William Butler & Raymond Hough
Drill-and-practice software
$40 (4-disk set)
John Wiley & Sons, Inc.
605 3rd Avenue
New York, NY 10158
(this software may not be available)
Diskette #1 Periodic Properties of the Elements
Diskette #2 Nomenclature and Oxidation Numbers
![]()
![]()