 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Thermochemistry
Chapter 4
Objectives
Following your study of this chapter, you should be able to
![]()
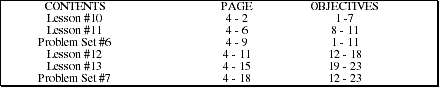
![]()
![]()

1a. Describe the chemical and physical changes observed in the two reactions which were demonstrated in lecture. Indicate the energy change which occurred in each reaction.

b) Define the term thermodynamics and explain how the term thermochemistry is related to thermodynamics.
![]()
![]()
2a. Distinguish between the meaning of the terms kinetic energy and potential energy.
![]()
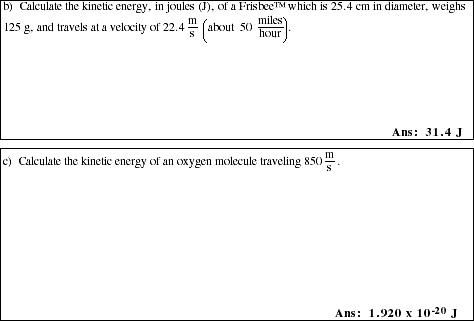
3. What is the SI unit for energy? State the unit conversion factor relating the SI unit to calories.
![]()
4. Distinguish between the meaning of the terms temperature and heat. Provide an example of
two objects that are at the same temperature, yet contain different amounts of heat.
![]()
![]()
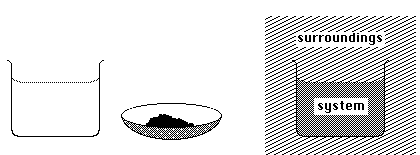
5. Identify the meaning of the terms system and surroundings for chemical reactions. Provide an example distinguishing between system and surroundings.
![]()
![]()
6. Define the terms exothermic and endothermic. (Using a sketch similar to that used in
lecture, show how heat flows between system and surrounding for both types of process.)
![]()
7a. Distinguish between the meaning of the terms specific heat and heat capacity. What would
be the relative temperature change for two substances of equal mass, but having different
specific heats, upon absorbing an equal amount of heat?
![]()
![]()

![]()
![]()

![]()
8. Label the important components of the coffee-cup calorimeter shown below. What
property of a chemical reaction does a coffee-cup calorimeter measure? What type of
reactions are studied?
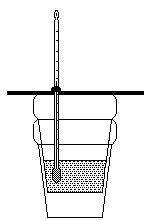
9a. Write the mathematical equation that relates the heat released by the chemical reaction to the heat absorbed by the water and the coffee-cup calorimeter.
![]()

![]()
![]()
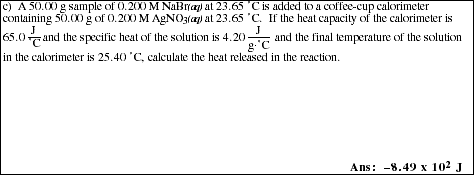
10. Label the important components of a bomb calorimeter shown below. What property of a chemical reaction does a bomb calorimeter measure? What type of reaction is studied?
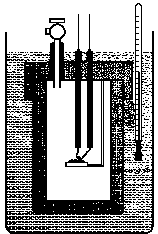
![]()
![]()
11a. Write the mathematical equation that relates the heat released by the chemical reaction to the heat absorbed by the water and the bomb calorimeter.
![]()
![]()
![]()
Problem Set #6
AP Chemistry by Satellite
ALL work must be shown to receive full credit.
PS6.1. Rank the following substances from lowest specific heat to highest specific heat.
C(s), Fe(s), N2(g), H2O(l), Hg(l)
![]()
PS6.2. Rank the following substances from lowest heat capacity to highest heat capacity.
1 kg H2O(l), 10 kg Cu(s) cube, 5 kg Fe(s) ball, 5 kg C(s) rod
![]()
PS6.3. Below is a table of specific heats for several different metals

![]()
Calculate the molar heat capacity
![]() for each of the metals. Based on the results
for each of the metals. Based on the results
of your calculations, estimate the specific heats of iron and silver.
![]()
![]()
PS6.4a. How much heat must be absorbed by a 75.0 g sample of water for the temperature to change from 24.5 ºC to 63.8 ºC?
![]()
b. Calculate the temperature change if a 75.0 g sample of zinc absorbed the same amount
of heat. The specific heat of zinc is listed in problem 6.3.
![]()
PS6.5. A 28.4 g sample of an unknown metal was heated to 110.0 ºC and plunged into a 100 g
sample of water initially at a temperature of 24.60 ºC. The final temperature of the
mixture was 25.34 ºC. Calculate the specific heat of the metal. Identify the metal.
![]()
PS6.6. When 1.000 g of KNO3 is dissolved in 120.0 g of water initially at 24.25 ºC in a
coffee-cup calorimeter, the final temperature is found to be 23.44 ºC. Calculate the heat
absorbed per gram and per mole when KNO3 dissolves in water. (Assume the heat
capacity of the calorimeter is zero.)
![]()
PS6.7. Calculate the heat produced per mole of aspirin when 2.216 g of C9H8O4 are reacted with
excess oxygen in a bomb calorimeter containing 4.40 kg of water. The temperature
change measured is 2.32 ºC. The heat capacity of the calorimeter is 2340 J/ ºC) .
![]()
![]()

12. State the first law of thermodynamics both in words and using a mathematical equation.
![]()
13a. What is the definition of the internal energy of a chemical system? Can the exact amount of
the internal energy be calculated for stable chemical systems? Can a change in internal
energy during a chemical reaction be calculated?
![]()
b) Given the chemical equation
![]()
And the diagram
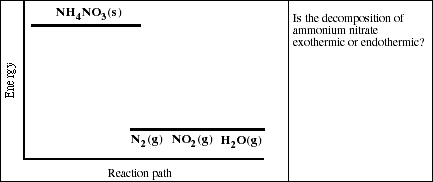
![]()
![]()
14. Write the mathematical equation that relates the internal energy change to heat flow and work.
![]()
15a. Rewrite equation in Exercise 14 for the special case of a constant volume reaction (as in a
bomb calorimeter).
![]()
b) Rewrite equation in Exercise 14 for the special case of a constant pressure reaction (as in a
coffee-cup calorimeter).
![]()
16. Define enthalpy in terms of internal energy.
![]()
17a. List the three important properties which are important when using enthalpy.
![]()
![]()
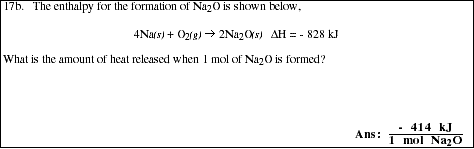
![]()

![]()
![]()
18. Define the term state function.
![]()
![]()

![]()
19a. Define Hess' Law.
![]()
![]()
![]()
20. Define the term standard state as it is used in chemical thermodynamics.
![]()
21. Define the term standard heat of formation and provide several examples of chemical
equations that characterize a formation reaction.
![]()
22a. Define the term heat of reaction and write the mathematical equation used to calculate the
heat of a chemical reaction.
![]()
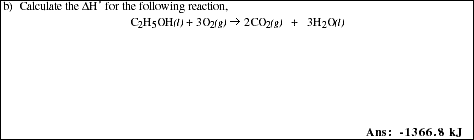
![]()
![]()

![]()

23. Define the term fuel value and list several compounds and their respective fuel value.
![]()
![]()
Problem Set #7
AP Chemistry by Satellite
ALL work must be shown to receive full credit.

![]()
is -683 kJ. Calculate the heat produced when 2.57 g of CaBr2 are formed.
![]()
PS7.3. For which of the following reactions is
![]() reasoning in each case.
reasoning in each case.
![]()
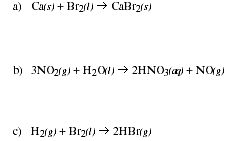
![]()
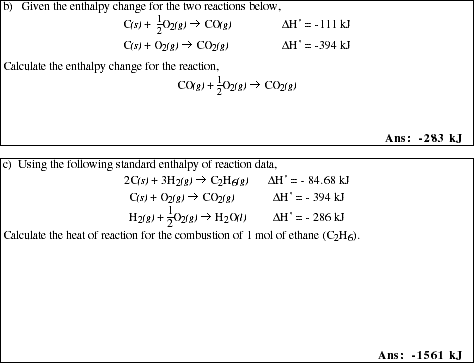
PS7.4. Given the enthalpy change for the two reactions
![]()
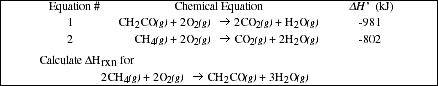
![]()
![]()
PS7.5. Given the following equations;
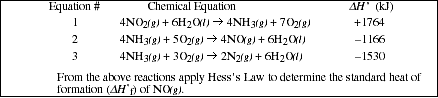
![]()
PS7.6. Using a table of Standard Enthalpies of Formation in your text or another reference
book, calculate the enthalpy of reaction for each of the following;
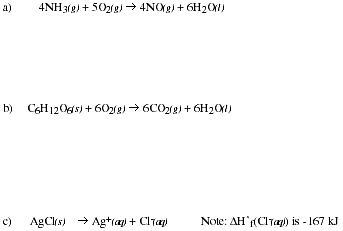
![]()
![]()
PS7.7. The molar heat of combustion of nitroethane, C2H5NO2(l), to CO2(g), H2O(l) and N2(g)
at 25 ºC is -1348 (kJ/mol). Determine the
![]() for nitroethane.
for nitroethane.
![]()
![]()
PS7.8a. Given the following thermodynamic data,

![]()
Calculate ![]() for 1 mol of CaCl2
dissolving in water. Is the dissolution of CaCl2
endothermic or exothermic?
for 1 mol of CaCl2
dissolving in water. Is the dissolution of CaCl2
endothermic or exothermic?
![]()
b. If 18.0 g of CaCl2 is added to 100. g of water initially at 23.5 ºC, calculate the
temperature of the solution after the CaCl2 dissolves. (Assume no heat is lost to the
container or the surroundings and the specific heat of the solution is the same as that of
water.)
![]()
![]()
PS7.8b. (Continued)
![]()
PS7.9. Determine the standard enthalpy of vaporization (transition from liquid to gas) for
CCl4(l).
![]()
![]()
Microcomputer software
![]()
Computer Aided Instruction for General Chemistry by William Butler & Raymond Hough
Drill-and-practice software
$40 (4-disk set)
John Wiley & Sons, Inc.
605 3rd Avenue
New York, NY 10158
(this software may not be available)
Diskette #3 Chemical Thermodynamics
![]()
![]()