 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Chemical Reactions and Stoichiometry
Chapter 3
Objectives
Following your study of this chapter, you should be able to

![]()
![]()

1. Explain how the process of balancing a chemical equation is necessary to satisfy the
law of conservation of mass.
![]()
2a. Where do integral (whole) numbers appear in balanced chemical equations?
![]()
b) Which numbers can be changed to balance the equation?
![]()
3a. Write out a useful sequence of steps for balancing any chemical equation.
![]()
![]()
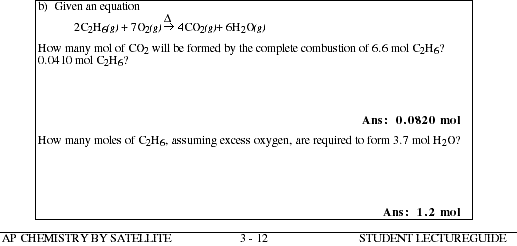
3b. In the following chemical equation
![]()
![]()
4. Write a chemical equation illustrating each of the following reaction types. In each
case, what are the important characteristics that could help you recognize other
chemical equations as belonging to that particular category?
a) formation reaction
![]()
b) combustion reaction
![]()
c) decomposition reaction
![]()
![]()
4. CONTINUED
d. single replacement reaction
![]()
e. double replacement reaction
![]()
f. neutralization reaction
![]()
5. Practice predicting the products of chemical reactions.
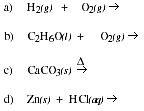
![]()
![]()
6. List the common acids and bases emphasized during the Lesson.

7. Write equations for the neutralization reactions described during the Lesson. What
are the products common to neutralization reactions?
![]()
8a. Write balanced chemical equations for the precipitation reactions described
during the Lesson. Briefly describe the change in appearance of the solution
that occurred in each case.
![]()
b) What class of reaction (see #4) do precipitation reactions belong to?
![]()
![]()

9. Define relative atomic mass unit (amu). What is the relationship between amu and
the mass of a single atom expressed in grams?
![]()
10. Describe how the formula mass of a compound is calculated.
![]()
11. Define the terms mole and Avogadro's number. How are these two quantities
related?
![]()
12a. Write a general mathematical equation that relates the number of moles of a
compound to the molar mass of that compound.
![]()
![]()
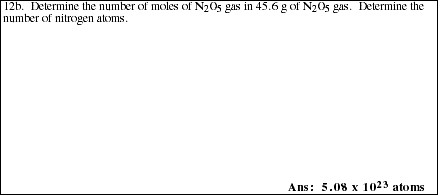
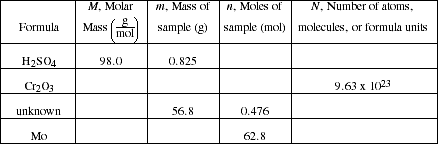
c) Complete the following table
13. Write a general mathematical equation for determining the percentage composition
of each element in a ternary compound. Use the symbols X, Y, and Z for the
elements and a,b, and c for the subscripts: XaYbZc.
![]()
![]()
14a. Briefly describe what information is contained in the formula of a compound. What
initial information must be available to determine the empirical formula for a
compound?
![]()

![]()
![]()
Problem Set #3
AP Chemistry by Satellite
![]()
ALL work must be shown to receive full credit.
PS3.1. Predict the products for each of the following chemical reactions and balance the
chemical equation.
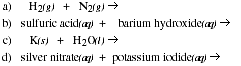
PS3.2. Determine the number of moles in each of the following.
a) 25.6 g of acetic acid (HC2H3O2)
![]()
b) 1.89 x 10-4 g of Ca3(PO4)2
![]()
PS3.3. Determine the number of oxygen atoms in each of the following.
a) 6.451 moles of C6H8O6 (vitamin C)
![]()
b) 1.89 x 10-4 g of Ca3(PO4)2
![]()
PS3.4. Determine the mass in grams in each of the following.
a) 0.0721 moles of H3PO4 (phosphoric acid)
![]()
b) 72 atoms of sulfur
![]()
PS3.5. Determine the percent composition of each element in Ni3(PO4)2.
![]()
![]()
PS3.6. Determine the empirical formula for a compound which is 26.6 % potassium,
35.4 % chromium and 38.1 % oxygen.
![]()
PS3.7. When a solid compound which is 27.62 % Ca, 22.06 % S, 49.62 % O and
0.700 % H is heated, a solid product is isolated which is 29.45 % Ca, 23.52 %
S and 47.03 % O. What is the other product formed? Write the chemical
equation which describes the reaction which has occurred.
![]()
![]()
PS3.8. Determine the simplest formula for gold chloride if a 0.303 g sample of gold
chloride reacts with AgNO3 to produce gold nitrate and 0.430 g of AgCl.
HINT: Given that 0.430 g of AgCl are formed in the reaction one must
assume all the chloride in 0.430 g of AgCl comes from the gold chloride.
![]()
![]()

15. Using the balanced chemical equation shown below, write the mole ratio of every
pair of reactant and product entities.
![]()
![]()
16a. List the general steps required to solve any problem in which you are given the
mass of each reactant (one reactant is in excess) and asked to calculate the mass of
one or more products formed as the result of a complete reaction.
![]()
![]()
![]()
![]()
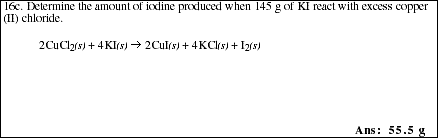
d) The equation for the reaction is
![]()
Consider a mixture of sulfur and oxygen in a closed container as illustrated below:
![]()
![]()
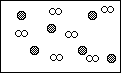
![]()
For each case explain why the representation is correct or incorrect.
![]()
![]()
![]()
17a. List the general steps required to solve any problem in which you are given the
mass of each reactant and asked to calculate the mass of one or more products
formed as the result of a complete reaction.
![]()

![]()
![]()

![]()
![]()
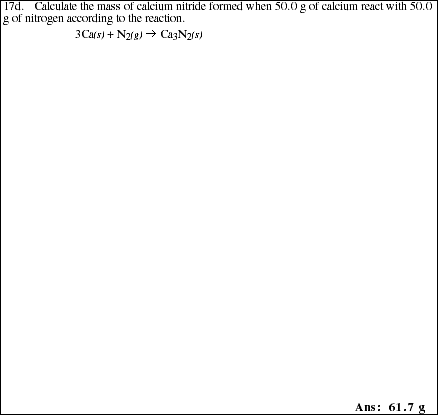
![]()
![]()
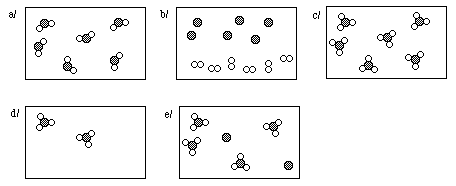
17e. The reaction of element X with element Y
![]()
![]()
is represented in the following diagram
![]()

![]()

![]()
Write a balanced chemical equation describing the reaction.
![]()
Identify the limiting reagent.
![]()
Complete the product side of the diagram below. Assume the same reaction will
occur as described above.
![]()

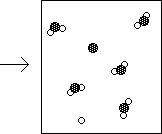
![]()
![]()

18. Define the term "percent yield."
![]()
![]()
Problem Set #4
AP Chemistry by Satellite
![]()
ALL work must be shown to receive full credit.
PS4.1. Gallium metal reacts with hydrogen chloride according to the equation
![]()
a) How many grams of hydrogen chloride are required to completely react with
24.4 g of gallium metal?
![]()
b) How many grams of gallium chloride are formed?
![]()
PS4.2. How many grams of gallium chloride are formed when 24.4 g of gallium metal
reacts with 40.0 g of hydrogen chloride?
![]()
PS4.3. In the formation reaction
![]()
Calculate the number of moles of C2H6 formed when
a) 2.0 moles of C are reacted with 5.0 moles of H2
![]()
![]()
4b. 6.0 moles of H2 are reacted with 4.0 moles C
![]()
c) 9.0 moles of H2 are reacted with 5.0 moles of C
![]()
d) 0.0812 moles of C reacted with 0.125 moles of H2
![]()
PS4.4. In the reaction
![]()
![]()
20.0 g of Hg are reacted with 5.00 g of O2. Calculate the maximum amount, in
grams, of HgO formed.
![]()
![]()
PS4.5. In the reaction
![]()
![]()
30.0 g of NH3 are reacted with 30.0 g of O2. Calculate the mass of H2O and
NO formed. What mass of the excess reactant remains unreacted?
![]()
PS4.6. A common laboratory method for determination of arsenic is described in the
reaction,
![]()
![]()
a) If 2.50 g of As2O3, 4.50 g of I2 and 4.00 g of H2O are mixed, and the
reaction proceeds to completion, which reactant is the limiting reagent?
![]()
![]()
6b) Calculate the mass of As2O5 which is theoretically possible.
![]()
c) If only 1.80 g of As2O
reaction. 5 is actually produced, determine the percent yield in the
![]()
PS4.7. An industrial method for the production of elemental zinc from its ore is to
'roast' the crude ore in oxygen and then react it with CO. The following
reactions describe the chemical process,
![]()
A 10.0 kg sample of an ore containing ZnS was chemically treated according to
the reactions described above producing 2.85 kg of Zn. Determine the
percentage of ZnS contained in the original sample of the ore.
![]()
![]()

![]()
19. Define the terms "solute" and "solvent".
![]()
20a. Define the terms concentration and molarity.
![]()
b) One of the two 500 mL beakers below contains a solution of a cyanide salt and the
other contains a solution of ethanol. What additional information would you like to
have before deciding which solution to drink?
![]()
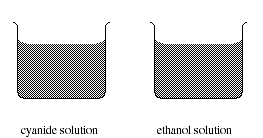
![]()
![]()
21a. Write the general mathematical equation for determining the molarity of a solution
given the moles of solute and the volume of solution.
![]()
b) Rearrange the above equation to solve for the moles of solute in terms of the
molarity of the solution and the volume of solution.
![]()
c) Rearrange the mathematical equation in exercise 21a. to solve for the volume of
solution in terms of the molarity of the solution and the moles of solute.
![]()

![]()
![]()

![]()
22a. How is the amount of substance (number of moles) of each reactant and product
measured in reactions that take place in solution?
![]()
![]()
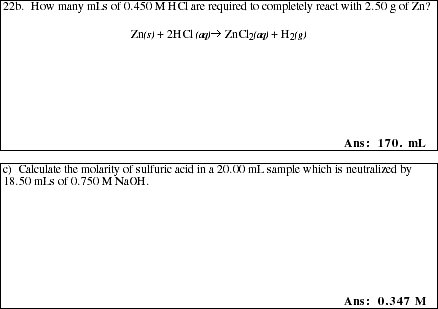
![]()
![]()

![]()
![]()
Problem Set #5
AP Chemistry by Satellite
ALL work must be shown to receive full credit.
PS5.1. Calculate the molarity of a solution containing,
a) 23.9 g of NaCl dissolved in enough water to have 300 mL of solution
![]()
b) 1.69 x 10-3 moles HCl in 12.0 mL of water
![]()
PS5.2. Describe how to prepare the following solutions.
a) 450 mL of 0.500 M NaOH, from solid NaOH
![]()
b) 900 mL of 1.00 M HCl, from 12.0 M HCl (concentrated HCl)
![]()
PS5.3. In the reaction,
![]()
How many grams of magnesium will react with 250. mL of 0.500 M HCl?
![]()
![]()
PS5.4. How many grams of H2 will be produced when 1.00 g of Mg is added to a
250. mL sample of 0.500 M HCl? (Use the chemical reaction in 5.3.)
![]()
![]()
PS5.5. Calculate the volume of 0.360 M NaOH needed to neutralize 27.0 mL of 0.820
M HCl. (Note: Write the chemical equation.)
![]()
PS5.6. Calculate the concentration of phosphoric acid produced when 2.00 g of P2O5
is added to 500 mL of distilled water. (Assume the final volume of the solution
is 500 mL.) The important reaction is,
![]()
![]()
How many moles of water remain unreacted?
![]()
![]()
PS5.7. Potassium dichromate in acidic solution is frequently used to determine the
concentration of Fe(II) in solution. The equation which describes the reaction
is:
![]()
![]()
A solution of Cr2O72- is prepared by dissolving 6.425 g of K2Cr2O
mL of water. (Assume no significant change in volume when the solution is7 in 800.0
prepared.) A total of 21.35 mL of this solution is required to reach the end-
point in a titration of a 250.0 mL sample containing Fe(II). Determine the
concentration of Fe(II) in the solution.
![]()
![]()
Microcomputer software
Project SERAPHIM
Dr. John Moore
Department of Chemistry
University of Wisconsin
Madison, WI 53706
AP304 MOLE CALCULATIONS
QUIZ ON MOLAR MASSES
MOLES IN SPACE
AP305 BALANCED EQUATIONS
MOLE DRILL
MOLE EXERCISE
MOLE DEMO
MOL-MOL TUTOR
AP306 STOICHIOMETRY
MOLE DRILL
LIMITING REAGENT
AP502 MOLARITY
![]()
Introduction to General Chemistry by Stan Smith, Ruth Chabay and Elizabeth Kean
Drill-and-practice software
$500 (10-disk set)
Falcon Software
P.O Box 200
Wentworth, NH 03282
1-603-764-5788
Diskette #3 Chemical Formulas and Equations
Diskette #4 Atomic Weights
Diskette #5 Percent Composition
![]()
Computer Aided Instruction for General Chemistry by William Butler & Raymond Hough
Drill-and-practice software
$40 (4-disk set)
John Wiley & Sons, Inc.
605 3rd Avenue
New York, NY 10158
(this software may not be available)
Diskette #1 Stoichiometry: Chemical Arithmetic
Equations and Molarity
![]()
![]()