AP Chemistry by Satellite Lectureguide
Student Edition
The Atom
Chapter 2
Objectives
Following your study of this chapter, you should be able to
- state the law of conservation of mass, constant composition and law of multiple
proportion and to use experimental data to verify the laws.
- tell the number of atoms of each element in a formula unit for a compound, given the
chemical formula. Write chemical formulas, given the number of atoms of each
constituent element in the formula unit of the compound.
- describe significant experiments that led to the current model of the atom and explain
how each contributed to the current understanding of atomic structure.
- describe mass differences and charge differences among electrons, protons and
neutrons.
- describe the arrangement of electrons, protons and neutrons in atoms.
- define isotope, atomic number and mass number in terms of the number of electrons,
protons and neutrons.
- calculate numbers of electrons, protons, and neutrons in an atom, given the atomic
number and the mass number.
- calculate the average atomic mass given the isotopic abundance.
- define the terms radioactivity and radioisotope
- distinguish between alpha, beta, and gamma radiation
- write symbols for alpha particle, beta particle (electron), proton, neutron, and
positron.
- write and balance nuclear equations representing alpha and beta decay
- distinguish between nuclear fission and nuclear fusion.
- identify those elements that form stable diatomic molecules.
- distinguish between the meaning of the terms atom, molecule and ion.
- locate groups of atoms in the periodic table using two different organizing schemes:
- metals, nonmetals and metalloids
- these named chemical families: alkali metals, alkaline earth metals, chalcogens,
halogens, noble gases
- identify the 'normal' ionic charge for the ions in the chemical families; alkali metals,
alkaline earth metals, chalcogens and halogens.
- write the chemical formula for a simple ionic compound given the name and charge
of both cation and anion.
- predict whether any given pair of elements will form an ionic or a covalent
compound.
- name simple ionic and covalent compounds, given the formula.
- write the formula for simple ionic and molecular compounds given the IUPAC name.




- Briefly state each of these laws.
-
a). Conservation of mass

b). Constant composition

c). Multiple proportion
 Determine the number of atoms of each element in the following chemical
Determine the number of atoms of each element in the following chemical
formulas.
-
a) Mg(OH)2
b) PCl5
c) C33H32N4O4Fe

d) The element composition for a single formula unit of a compound was determined
to be, one cobalt atom, two chlorine atoms, twelve hydrogen atoms and six oxygen
atoms. Write the chemical formula for this compound.

- Briefly describe each of the following scientist's contribution to the growth of
chemistry.
-
a) Joseph Louis Gay-Lussac

b) J. J. Thomson

c) Robert Millikan

d) Henri Becquerel

e) Ernest Rutherford

f) Dmitri Mendeleev

g) H. G. J. Moseley

- Complete the following table.

- Identify the location of electrons, protons and neutrons in an atom.
 Define the terms isotope, atomic number and mass number.
Define the terms isotope, atomic number and mass number.
-

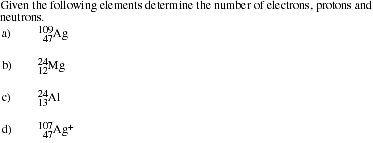


-
8a. Define the term atomic mass unit including the basis for measurement of atomic
mass and write the general mathematical equation for solving for the average
atomic mass of any element, given the atomic mass of each isotope and its percent
abundance.



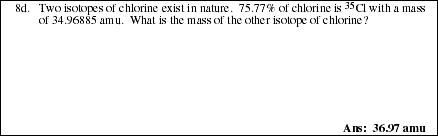
-
9a. Define radioactivity and briefly discuss the circumstances under which it was first
observed.

b) What is a radioisotope? What factors determine whether or not a particular
isotope will be radioactive?

- Distinguish between alpha, beta, and gamma radiation in terms of size, mass,
charge, and ability to penetrate matter.
 Write the symbols for the species listed below. Explain what the numbers used as
Write the symbols for the species listed below. Explain what the numbers used as
superscripts and subscripts tell us and how they are used in balancing nuclear
equations.
-
alpha beta
proton neutron
electron positron
gamma

12a. Explain what happens when a nucleus undergoes alpha decay.

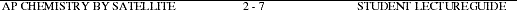
-
- b. Complete and balance the following nuclear equations.
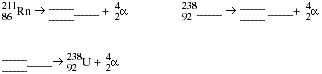
 Explain what happens when a nucleus undergoes beta decay.
Explain what happens when a nucleus undergoes beta decay.
 Complete and balance the following nuclear equations.
Complete and balance the following nuclear equations.
 Explain the difference between nuclear fission and nuclear fusion. Give an
Explain the difference between nuclear fission and nuclear fusion. Give an
example of a fission reaction and a fusion reaction.


- List the name and chemical formula for each of the elements that exist as diatomic
elements.
 Identify an element whose standard state molecular form contains four atoms?
Identify an element whose standard state molecular form contains four atoms?
eight atoms?
 Distinguish between atoms, molecules and ions by listing the unique properties of
Distinguish between atoms, molecules and ions by listing the unique properties of
each that are not shared by the others.
 Write the names and symbols of, at least, two examples of elements belonging to;
Write the names and symbols of, at least, two examples of elements belonging to;
metals, alkaline earth metals, metalloids, halogens, alkali metals, chalcogens, and
nonmetals.

-
-
a. Do the elements in the alkali metals group, the alkaline earth metals group and the
additional metals aluminum and gallium form positive or negative ions? What is
the normal ionic charge for each group?

b) Do the elements in the halogen group, and the element oxygen form positive or
negative ions (primarily)? What is the normal ionic charge for each group?
 What is the important criterion, in terms of ionic charge, for determining the
What is the important criterion, in terms of ionic charge, for determining the
formula for a compound containing a metallic element and a nonmetallic element?
 Identify the class(es) of elements found in the majority of ionic compounds? of
Identify the class(es) of elements found in the majority of ionic compounds? of
covalent compounds?

-
-
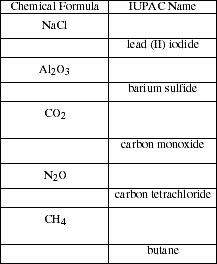
a. List the rules for naming the common binary ionic compounds. Name the
compounds listed.

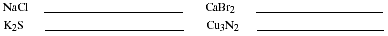

-
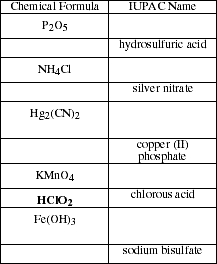
- b. List the rules for naming the common polyatomic cations and anions. Name the
ionic compounds listed.

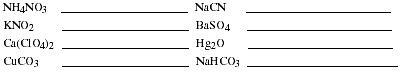
c) List the general rules for naming binary covalent compounds. Name the
compounds listed.

-
-
d. continued.
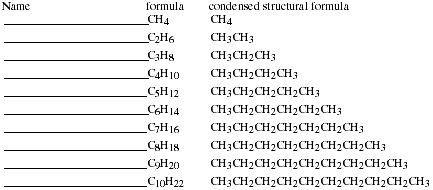
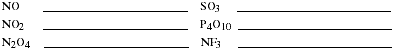 e) Binary compounds of carbon and hydrogen (hydrocarbons) do not follow the
e) Binary compounds of carbon and hydrogen (hydrocarbons) do not follow the
general rules of nomenclature for covalent compounds. Write the names of the
simple hydrocarbons listed below.

f) Acids are a very important class of compounds which also have special rules of
nomenclature. Define the term acid and then list the rules for naming acids.
Write the names of the compounds listed.

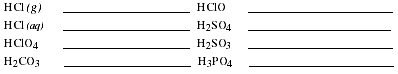

- Practice naming compounds given their formula and writing the formula of a
compound given its name.

AP Chemistry by Satellite
Problem Set #2
ALL work must be shown in Problems 1 - 10 full credit.
PS2.1. Sketch a neutral fluorine atom indicating the proper number and approximate
location of protons, neutrons and electrons.

PS2.2. How many electrons would be needed to equal the mass of a single proton?

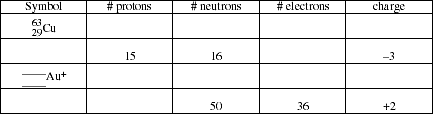
PS2.3. Complete the following table
PS2.4. Copper exists in nature in two isotopic forms with masses 62.96 u and 64.96
u. The accepted atomic mass for copper is 63.55 u. Determine the percent
abundance for each isotope. How many grams of each isotope would be found
in a 10.0 g sample of pure copper metal?

PS2.4. (Continued)

PS2.5 Write a balanced nuclear equation for each of the following transformations.
a).
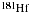 undergoes beta decay.
undergoes beta decay.

b) Radium-226 decays to a radon isotope.

c). Neptunium-233 undergoes alpha decay.

d). Two 21H atoms undergo a fusion reaction.

e). Uranium-235 absorbs a neutron and undergoes fission producing barium-141,
3 neutrons, and another nucleus.

PS2.6. Predict formulas for each of the following combinations. Write the name of
each compound formed. (Note: One of the compounds has two correct
formulas and four correct names!)
-
a) gallium and oxygen
b) magnesium and sulfate
c) aluminum and chlorine
d) calcium and phosphate
e) iron and nitrate

PS2.7a. The simple hydrocarbons listed in exercise 20e. have the same general
formula. Complete the formula where n is the number of carbon atoms.
CnH?

PS2.7b. For molecules having more than three carbon atoms, the straight chain
arrangement shown in exercise 20e. is not the only possible arrangement of
atoms. For example, the molecular formula C5H12 has three possible
structures. Write the condensed molecular formula or draw the structure for
each of these compounds and name each one.
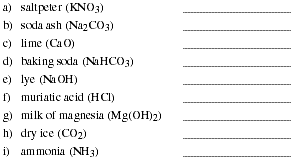
PS2.8. Many familiar substances have common, unsystematic names. In each of the
following cass, give the correct systematic name:
PS2.9. Write the chemical formula of each substance mentioned in the following
word descriptions.
a) Zinc carbonate can be heated to form zinc oxide and carbon dioxide.

b) On treatment with hydrofloric acid, silicon dioxide forms silicon
tetrafluoride and water.

c) Sulfur dioxide reacts with water to form sulfurous acid.

d) The substance hydrogen phosphide is commonly called phosphine.

e) Perchloric acid reacts with cadmium to form cadmium (II) perchlorate.

f) Vanadium (III) bromide is a colored solid.

PS2.10. Using the CRC Handbook of Chemistry and Physics, find the density, melting
point, boiling point and the phase at room temperature for CF4, CCl4, CBr4
and CI4.

Microcomputer software
Project SERAPHIM
Dr. John Moore
Department of Chemistry
University of Wisconsin
Madison, WI 53706
AP301 NAME THE IONS
AP303 NAMING


Introduction to General Chemistry by Stan Smith, Ruth Chabay and Elizabeth Kean
Drill-and-practice software
$500 (10-disk set)
Falcon Software
P.O Box 200
Wentworth, NH 03282
1-603-764-5788
Diskette #1 The Elements
Diskette #2 Inorganic Nomenclature
Diskette #4 Atomic Weights


 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index![]()

![]()
![]()

![]()
![]()
![]()
![]()

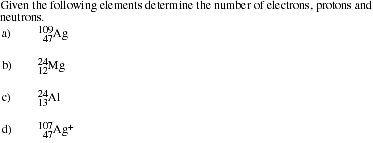
![]()
![]()

![]()

![]()
![]()
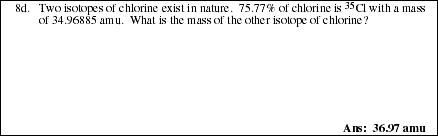
![]()
![]()
![]()
![]()
![]()
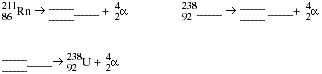
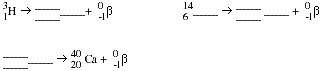
![]()
![]()

 Identify an element whose standard state molecular form contains four atoms?
Identify an element whose standard state molecular form contains four atoms?![]()
![]()
![]()
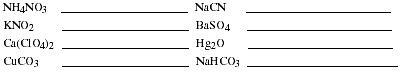
![]()
![]()
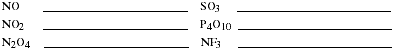 e) Binary compounds of carbon and hydrogen (hydrocarbons) do not follow the
e) Binary compounds of carbon and hydrogen (hydrocarbons) do not follow the
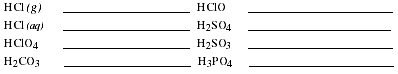
![]()
![]()


![]()
![]()
![]()
![]()

![]()
![]()
![]()
![]() undergoes beta decay.
undergoes beta decay.
![]()
![]()
![]()
![]()
![]()
![]()

![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()