 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Oxidation and Reduction
Chapter 18
Objectives
Following your study of this chapter, you should be able to
![]()

![]()
![]()

1a. Identify each of the chemical equations below as to the reaction type (combustion, formation, or decomposition).
![]()
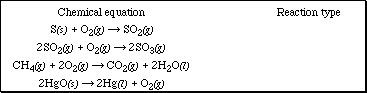
b. Identify two characteristics common to these equations.
![]()
c. Explain the historic interpretation of oxidation and reduction in chemical
reactions.
![]()
d. Define the terms oxidation, reduction, oxidizing agent and reducing agent.
![]()
![]()
2. Summarize the steps required to balance an oxidation-reduction reaction in aqueous solution.
![]()
a. Balance the following oxidation-reduction equation
![]()
![]()
![]()
b. Balance the following oxidation-reduction equation
![]()
![]()
![]()
![]()
c. Balance the following oxidation-reduction equation
![]()
![]()
![]()

3a. Describe the oxidation-reduction reaction demonstrated in lecture and write the two half-reactions and the overall chemical equation.
![]()
b. Complete the sketch of the electrochemical cell shown below. In your sketch,
identify the important components of the cell, i.e. the anode and cathode
electrodes, the ions in solution in the anode and cathode compartments, and the
salt bridge. In addition, indicate the direction of flow of electrons in the wire, and
the direction of flow of ions in the salt bridge, the anode and the cathode
compartments.
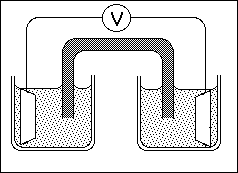
c. Based on the lecture discussion, describe the reaction which is occurring at the surface of the anode electrode and the cathode electrode.
![]()
![]()
3. (continued)
d. Explain why the cations in the salt bridge migrate towards the cathode compartment and why the anions in the salt bridge migrate towards the anode compartment.
![]()
4a. Write the shorthand cell notation for the electrochemical cell constructed in
lecture.
![]()
b. Write the oxidation-reduction equation and sketch the electrochemical cell using
the following electrochemical cell notation.
![]()
![]()
![]()
b. Write the oxidation-reduction equation and sketch the electrochemical cell using the following electrochemical cell notation.
![]()
![]()
![]()
 5a. Define the term electromotive force and the unit volt.
5a. Define the term electromotive force and the unit volt.
![]()
b. How is the emf in an electrochemical cell measured?
![]()
6a. Define the term standard emf and explain the importance of a reference half-
reaction. Write the reference half-reaction discussed in lecture and its emf.
![]()
![]()
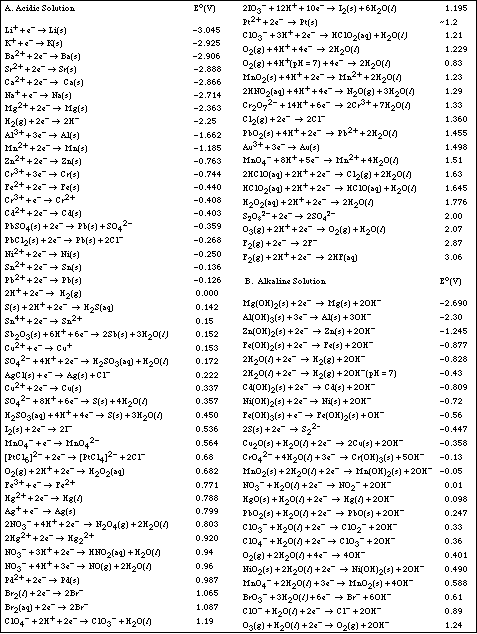
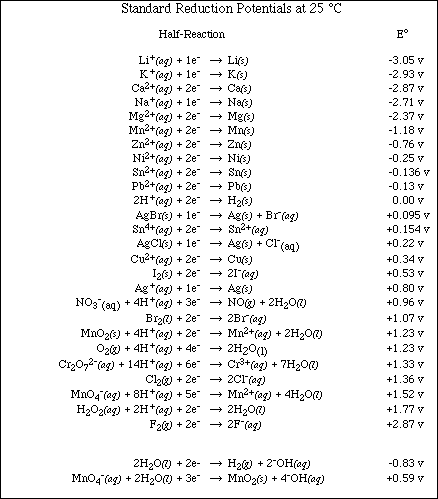
b. Using the table of Standard Reduction Potential shown below, identify,
![]()
i) the substance most likely to be oxidized
ii) the substance most likely to be reduced
iii) the strongest oxidizing agent
iv) the strongest reducing agent
![]()
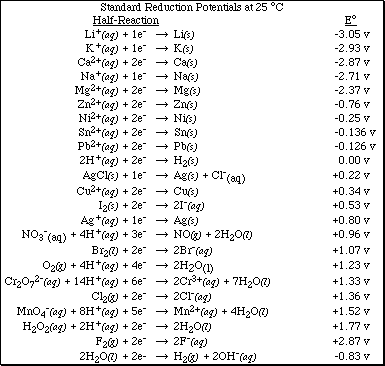
![]()
![]()
7. Using the table of Standard Reduction Potential complete the following problems.
![]()
a. Which of the following species is the strongest oxidizing agent, MnO4- (in acidic
solution), Br2(l), or Ca2+(aq)?
![]()
Ans: MnO4-(aq) in acidic solution
![]()
b. Will aluminum displace Cu2+ ion from an aqueous solution of Cu(NO3)2?
![]()
Ans: Yes
![]()
c. Will Mg displace Sn2+ from an aqueous solution of tin (II) nitrate?
![]()
Ans: Yes
![]()
![]()
![]()
d. Will lead metal dissolve in 1 M HCl?
![]()
Ans: Yes
![]()
e. From the following information estimate the ED for
![]()
![]()
![]()
i)the metal, M, dissolves in 1 M HNO3 but not in 1 M HCl. It will displace
Ag+(aq), but not Cu2+(aq).
![]()
Ans: Eº = -0.337 to -0.799 v
![]()
![]()
Problem Set #31
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
PS31.1. Balance the following oxidation-reduction reactions using the half-reaction method.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
PS31.2. Draw a diagram of the cells in which the following reactions occur. In each case, label the anode and cathode, the anode and cathode electrode material, the half-reaction at each electrode, the ions in the anode and cathode compartments and salt bridge, the direction of electron flow, and the direction of ion movement.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
PS31.3. Write the balanced chemical equation for the overall cell reaction represented in each of the following cell notations.
![]()
(a) Sn(s)|Sn2+(aq)||Ag+(aq)|Ag(s)
![]()
(b) Zn(s)|Zn2+(aq)||(Pt(s))Cl2(g)|Cl-(aq)
![]()
(c) Zn(s)|Zn2+(aq)||Cr2O72-(aq), H+(aq), Cr3+(aq)|Pt(s)
![]()
PS31.4. Which of the following species are reduced by Ag?
![]()
Cl2, Fe3+, Pb2+, I2, NO3- (in H+)
![]()
PS31.5. Which of the following species are oxidized by nitrate in acidic solution?
![]()
Cl-, Fe2+, Cu, Au, H+
![]()
![]()
PS31.6. Select a suitable species for each of the following
![]()
(a) an oxidizing agent able to convert Sn to Sn2+, but not Cu to Cu2+.
![]()
(b) a reducing agent capable of converting H+ to H2, but not Zn2+ to Zn.
![]()
(c) an oxidizing agent capable of converting Cl- to Cl2, but not F- to F2.
![]()
![]()

f. Determine whether the following reactions are spontaneous under standard conditions.
![]()
![]()
Ans: Not spontaneous as written.
![]()
![]()
Ans: Spontaneous as written.
g. Predict the products of the following reactions.
![]()
![]()
![]()
![]()
![]()
ii) Ag(s) + Br2(l) 
![]()
![]()
![]()
![]()
![]()
Ans: No reaction
![]()
![]()
![]()
Ans: No reaction
![]()
8a. Write the mathematical equation which relates the standard cell potential, Eº, for a
chemical reaction with its free energy, DGº, and with the equilibrium constant, K.
![]()
![]()
b. Calculate the standard free energy change and the equilibrium constant for the following reactions.
![]()
![]()
Ans: K = 6.36 x 1075
![]()
![]()
![]()
Ans: K = 1.58 x 1063
![]()
![]()
c. Given the following two half-reactions, determine whether H2O2 is unstable
with respect to water and oxygen.
![]()
![]()
![]()
Ans: DGº = -210 kJ
![]()
d. Given the following two half-reactions, calculate the magnitude of the solubility
equilibrium constant for AgCl(s).
![]()
![]()
Ans: K = 1.55 x 10-10
![]()
![]()

![]()
10. Complete the following problems.
![]()
a. Calculate Eº for the reaction
![]()
![]()
![]()
Ans: Eº = +1.10 v
![]()
i) Calculate Ecell when the ratio of these concentrations is small, that is, if
[Zn2+] = 1 x 10-4 M and [Cu2+] = 1.0 M.
![]()
Ans: Eº = +1.22 v
![]()
![]()
ii) Calculate Ecell when the ratio of these concentrations is small, that is, if [Cu2+] = 1 x 10-4 M and [Zn2+] = 1.0 M.
![]()
Ans: Eº = +0.98 v
b. Which of the following oxidizing agents become stronger as the [H+] is
increased? Which are unchanged? Which become weaker?
![]()
i) Br2
![]()
Ans: unaffected
ii) Fe3+
![]()
iii) MnO4-
![]()
Ans: increased
iv) H+
![]()
v) Cr2O72-
![]()
![]()
c. Calculate Ecell for the following system
![]()
Cu(s)|Cu2+(aq) (3.00 M) || Cu2+(aq) (0.100 M)|Cu(s)
![]()
Ans: Ecell = -0.0436 volt
![]()
![]()
 11a. Write the chemical equation which describes the discharge reaction in an alkaline
dry cell battery used in flashlights or transistor radios.
11a. Write the chemical equation which describes the discharge reaction in an alkaline
dry cell battery used in flashlights or transistor radios.
![]()
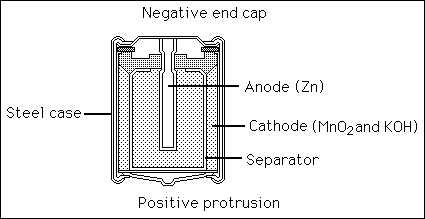
![]()
b. Write the chemical equation which describes the discharge reaction in an lead-
acid battery used in automobiles.
![]()
![]()
12a. Write the chemical equations involved in the corrosion of iron.
![]()
b. Sketch a simple diagram showing the location of the two half-reactions involved
in the corrosion of iron.
![]()
13. Distinguish between a voltaic cell and an electrolytic cell.
![]()
![]()
Problem Set #32
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
![]()
PS32.1. Calculate Eº and determine which of the following reactions will occur in the
forward direction under standard conditions? Balance the equations in acid solution.
![]()
![]()
![]()
![]()
![]()
![]()
PS32.2. Use standard reduction potentials to predict the spontaneous reaction, if
any, that occurs between the following. If no spontaneous reaction occurs, write NR.
![]()
![]()
![]()
![]()
![]()
PS32.2. (Continued)
![]()
![]()
![]()
![]()
![]()
![]()
PS32.3. Calculate DGº and K for the spontaneous reactions in problem 32.2.
![]()
![]()
![]()
![]()
PS32.3. (Continued)
![]()
![]()
PS32.4. Calculate the solubility product constant for the iron(II) hydroxide, given the
following information.
![]()
![]()
![]()
PS32.5. Calculate Ecell of the following cells at 25 ºC.
![]()
(a) Cr(s)|Cr3+(1.0 x 10-2 M)||Ni2+(2.0 M)|Ni(s)
![]()
(b) Zn(s)|Zn2+(1.0 x 10-2 M)||Zn2+(1.0 M)|Zn(s)
![]()
(c) Fe(s)|Fe2+(2.0 M)||Cr2O72-(1.0 M), H+(3.0 x 10-3 M), Cr3+(0.500 M)|Zn(s)
![]()
![]()
PS32.6. A galvanic cell consisting of a Cu versus a hydrogen electrode was used to determine the pH of an unknown solution. The unknown was placed in the hydrogen electrode compartment and the pressure of the hydrogen gas was controlled at 1 atm. The concentration of Cu2+ was 1 M and the E(not standard E) of the cell at 25 ºC was determined to be +0.48 V. Calculate the pH of the solution.
![]()
![]()
![]()
PS32.7. Consider the following half-reaction
![]()
![]()
Eº = +0.96 volts. Calculate Ered when NO3- is 1 M and NO is 1 atm and the pH is;
![]()
(a) 0
![]()
(b) 2
![]()
![]()
(c) 7
![]()
PS32.8. An iron ore sample weighting 0.8500 g is dissolved in HCl(aq), treated with
a reducing agent to convert all the iron to Fe2+(aq), and then titrated with
exactly 28.28 mL of 0.0652 M K2Cr2O7. What is the percent Fe, by mass,
in the ore sample?
![]()
![]()
![]()
![]()

![]()
14a. Write the chemical equation for the industrial preparation of Cl2(g) in the absence
of water.
![]()
b. Write the chemical equation which describes the reaction which occurs when
NaCl is electrolyzed in water.
![]()
c. Explain why different products are formed in the electrolysis of NaCl(l) and
NaCl(aq).
![]()
![]()
d. Predict the products when the following solutions are electrolyzed between inert electrodes.
![]()
i) Na2SO4(aq)
![]()
ii) CuSO4(aq)
![]()
![]()
iii) KI(s)
![]()
![]()

15. Define the term coulomb, ampere, and Faraday.
![]()
16. Complete the following problems:
![]()
a. What weight of cadmium will be deposited from a CdCl2 solution by passage of
a current of 1.50 amps for 30.0 minutes?
![]()
Ans: 1.57 g Cd
![]()
![]()
b. Consider an electrolysis that results in the production of 1.00 g of Cu metal from CuSO4.
![]()
i) How many Faradays of electricity are required?
![]()
Ans: 0.0314 F
![]()
ii) How many coulombs of electricity are required?
![]()
Ans: 3.03 x 103 C
![]()
iii) If the reduction takes 1 hour, how many amps are required?
![]()
Ans: 0.842 amp
![]()
![]()
Problem Set #33
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
![]()
PS33.1. When a sample of molten MgCl2 and a sample of aqueous MgCl2 are
electrolyzed with inert electrodes different products are obtained. Determine
the product of the electrolysis of each sample and explain why there is a
difference.
![]()
PS33.2a) A sample of molten CdCl2 and a sample of aqueous CdCl2 are
electrolyzed with inert electrodes. Determine the products of the
electrolysis of each sample.
![]()
b) If the sample of aqueous CdCl2 is electrolyzed using a cadmium metal
anode and an iron metal cathode what are the expected results at each
electrode?
![]()
![]()
PS33.3. Sketch a cell which depicts the electrolysis of a 1.00 M aqueous solution of HCl. Assume both the electrodes are copper. Indicate the ions in the solution and the half-reaction at each electrode. Calculate the minimum voltage required for the electrolysis to occur.
![]()
PS33.4. In the electrolysis of KCl(aq), how many liters of Cl2(g) are formed,
measured at STP, by a current of 10.0 amps for 1.5 hours? How many
moles of KOH are formed in the same period?
![]()
PS33.5. If an aqueous solution containing Pb2+ ions is electrolyzed using a current of
0.900 amps, calculate the mass of Pb(s) plated out after 50 minutes.
![]()
![]()
PS33.6. A certain quantity of electricity is passed through a series of solutions containing AgNO3, CrCl3, ZnSO4 and CuSO4.
![]()
a) If 1.00 g of Ag(s) was deposited in the first solution, how many grams of
metal were deposited in each of the remaining solutions?
![]()
b) How much electricity (in coulombs) was used?
![]()
c) If the process took place in 3.00 hours, what was the current produced
by the power source?
![]()
PS33.7. Can you electrolyze water into H2(g) and O2(g) using the following power
sources (theoretically)?
![]()
a) standard car battery
b) transistor radio battery
c) lantern battery
d) 'D' cell flashlight battery
e) Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s)
![]()
![]()
PS33.8. An iron object must be coated with another metal to protect it from corrosion. Rank the following metals from most protective to least protective to iron.
Al, Mg, K, Cu, Ag, Sn, Zn
Which metal would be the most appropriate choice (consider reactivity and cost) for an iron tool that will be exposed to the atmosphere?
![]()
![]()
![]()
![]()
![]()
![]()
![]()