 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Equilibria in Aqueous Solution
Chapter 16
Objectives
Following your study of this chapter, you should be able to
![]()
![]()
![]()

1a. Based on the lecture demonstration complete the following table.
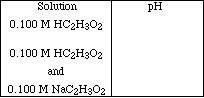
b. Define the term common ion effect.
![]()
c. Provide an example of an aqueous solution containing a weak acid and the
soluble salt of the acid.
![]()
d. Provide an example of an aqueous solution containing a weak base and the
soluble salt of the base.
![]()
e. How is the extent of dissociation of a weak acid or weak base affected by the
presence of its soluble salt?
![]()
![]()
f. Calculate the pH of a solution which is 0.53 M HC6H4NO2 and 0.50 M NaC6H4NO2.
![]()
Ans: pH = 4.82
![]()
g. Calculate the pH of a solution which is 0.245 M NH3 and 0.245 M NH4NO3.
![]()
Ans: pH = 9.15
![]()
![]()
2. Write the ionic and net ionic equations for the following reactions, the equilibrium expression for the net ionic equation, and determine the magnitude of the equilibrium constant.
![]()
![]()
![]()
![]()
![]()
![]()
What is the general conclusion which can be made from the magnitude of the
equilibrium constant in all three examples above?
![]()
![]()

3. Qualitatively, describe how the pH of a solution of a strong acid changes when a solution of strong base is added to it.
![]()
a. A titration is performed by adding 0.600 M KOH to 40.0 mL of 0.800 M HCl.
i) Calculate the pH before addition of any KOH.
![]()
Ans: pH = 0.097
![]()
ii) Calculate the pH after the addition of 5.0 mL of the base.
![]()
Ans: pH = 0.191
![]()
![]()
ii) (Continued)
Calculate the pH after the addition of 20.0 mL of the base.
![]()
Ans: pH = 0.478
![]()
Calculate the pH after the addition of 40.0 mL of the base.
![]()
Ans: pH = 1.00
![]()
Calculate the pH after the addition of 52.0 mL of the base.
![]()
Ans: pH = 2.06
![]()
Calculate the pH after the addition of 53.0 mL of the base.
![]()
Ans: pH = 2.67
![]()
![]()
iii) Calculate the volume of base needed to reach the equivalence point.
![]()
Ans: Vbase = 53.3 mL
![]()
iv) What is the pH at the equivalence point?
![]()
Ans: pH = 7.00
![]()
v) Calculate the pH after adding 5.00 mL of NaOH past the equivalence point.
![]()
Ans: pH = 12.48
![]()
![]()
4. Using the designated space below sketch the titration curve for each of the following cases.
a) 50.0 mL of 0.100 M HCl and 0.100 M NaOH
b) 50.0 mL of 0.00100 M HCl and 0.00100 M NaOH
Plot both curves on the graph below.
![]()
![]()

5a. Qualitatively, describe how the pH of a solution of a weak acid changes when a solution of strong base is added to it.
![]()
b. A titration is performed by adding 0.200 M NaOH to 24 mL of 0.350 M HOCl.
i) Calculate the pH before addition of any NaOH.
![]()
Ans: pH = 3.99
![]()
ii) Calculate the pH after the addition of 5.0 mL of the base.
![]()
![]()
ii) Calculate the pH after the addition of 5.0 mL of the base. (continued)
![]()
Ans: pH = 6.65
![]()
Calculate the pH after the addition of 15.0 mL of the base.
![]()
Ans: pH = 7.27
![]()
Calculate the pH after the addition of 25.0 mL of the base.
![]()
![]()
Calculate the pH after the addition of 25.0 mL of the base. (Continued)
![]()
Ans: pH = 7.69
![]()
Calculate the pH after the addition of 35.0 mL of the base.
![]()
Ans: pH = 8.22
![]()
Calculate the pH after the addition of 40.0 mL of the base.
![]()
![]()
Calculate the pH after the addition of 40.0 mL of the base. (Continued)
![]()
Ans: pH = 8.82
![]()
iii) Calculate the volume of base needed to reach the equivalence point.
![]()
Ans: Vbase = 42.0 mL
![]()
iv) What is the pH at the equivalence point?
![]()
Ans: pH = 10.31
![]()
![]()
v) Calculate the pH after adding 5.00 mL of NaOH past the equivalence point.
![]()
Ans: pH = 12.15
![]()
6. Using the designated space below, draw the titration curve for each of the
following cases.
![]()
a) 50.0 mL of 1.00 M HC2H3O2 and 1.00 M NaOH
b) 50.0 mL of 0.0100 M HC2H3O2 and 0.0100 M NaOH
![]()
![]()
![]()
![]()
Problem Set #28
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
PS28.1. Determine the pH for a solution containing the following substances+ .
![]()
a) 0.600 M HC3H5O3 and 0.600 M NaC3H5O3
![]()
b) 0.200 M HC3H5O3 and 0.200 M NaC3H5O3
![]()
c) 0.400 M NH4Cl and 0.811 M NH3
![]()
![]()
+ Important equilibrium constants are located in Appendix E.1 and E.2 on pages 979 and 980 of Brown and
LeMay.
![]()
![]()
d) 0.200 M HBr and 1.00 M HC3H5O2
![]()
e) 0.835 M C6H5NH3NO3 and 0.255 M C6H5NH2
![]()
PS28.2. Determine the magnitude of the equilibrium constant for the following
reactions
![]()
![]()
![]()
![]()
(Note: write the net ionic equation)
![]()
![]()
![]()
![]()
![]()
![]()
PS28.3. A titration is performed by adding 0.400 M KOH to 40.0 mL of 0.300 M HCl.
a) Calculate the pH before addition of any KOH.
![]()
b) Calculate the pH after the addition of 5.0, 20.0 and 29.5 mL of the base.
![]()
c) Calculate the volume of base needed to reach the equivalence point.
![]()
![]()
d) Calculate the pH at the equivalence point.
![]()
e) Calculate the pH after adding 5.00 mL of KOH past the endpoint.
![]()
f) Plot pH (y axis) versus volume of KOH added (x axis) for each calculation
above. Sketch the titration curve.
![]()
![]()
PS28.4. A titration is performed by adding 0.200 M NaOH to 24 mL of 0.350 M HC2H3O2.
![]()
a) Calculate the pH before addition of any NaOH.
![]()
b) Calculate the pH after the addition of 5.0, 25.0, and 40.0 mL of the base.
![]()
c) Calculate the volume of base needed to reach the equivalence point.
![]()
d) Calculate the pH at the equivalence point.
![]()
![]()
e) Calculate the pH after adding 5.00 mL of NaOH past the endpoint.
![]()
f) Plot pH (y axis) verses volume of NaOH added (x axis) for each calculation
above. Sketch the titration curve.
![]()

![]()
![]()
PS28.5. Calculate the pH at the equivalence point when 22.0 mL of 0.200 M hydroxylamine, HONH2, is titrated with 0.150 M HCl.
![]()
PS28.6. Calculate the pH of a solution prepared by mixing
a) 25.0 mL of 0.212 M NaOH and 36.0 mL of 0.187 M HCl
![]()
![]()
b) 125 mL of 0.345 M KOH and 87.0 mL of 0.400 M HC3H5O2
![]()
c) 400 mL of 0.100 M NH3 and 250 mL of 0.120 M HCl
![]()
![]()

7a. Define the term buffer.
![]()
b. Describe the characteristic behavior of a buffer when a small amount of acid is
added.
![]()
c. Write a chemical equation which describes the chemical reaction which occurs
when a strong acid is added to a solution containing the general buffer
![]()
![]()
d. Describe the characteristic behavior of a buffer when a small amount of base is
added.
![]()
e. Write a chemical equation which describes the chemical reaction which occurs
when a strong base is added to a solution containing the general buffer
![]()
![]()
![]()
8. Complete the following problems:
![]()
a. Calculate the pH of a solution prepared by mixing 20.0 mL of 0.300 M
HC2H3O2 with 20.0 mL of 0.350 M NaC2H3O2.
![]()
Ans: pH = 4.81
![]()
b. Specify the reagents and the specific concentrations of each reagent needed to
prepare a buffer solution which would have a pH of 4.19.
![]()
![]()

9. Complete the following problems:
a. Calculate the pH after adding 0.00200 moles of NaOH to the buffer in 8a.
![]()
Ans: pH = 5.10
![]()
![]()
b. Calculate the pH after adding 0.00200 mole of HCl to a new sample of the buffer in 8a.
![]()
Ans: pH = 4.54
![]()
![]()

10a. Define the terms precipitation and solubility.
![]()
b. Write a chemical equation and a net ionic equation for a reaction which forms a
precipitate.
![]()
c. Write the solubility equation for the precipitate formed above.
![]()
11a. Using the solubility table below predict whether the following compounds are
soluble or insoluble in water.
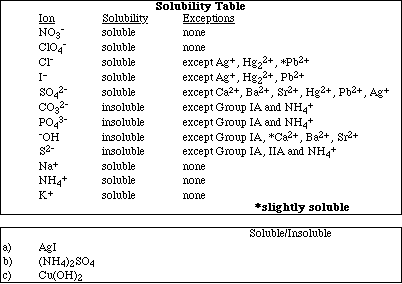
![]()
![]()
12. Complete and balance the following reactions. Identify all products phases as either (g)as, (l)iquid, (s)olid or (aq)ueous. If no reaction occurs, write NR.
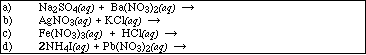
13. Write the equilibrium expression for the reaction described by the general solubility equation below,
![]()
![]()
14. Define the term solubility product constant.
![]()
15. Complete the following problem:
![]()
a. Calculate the Ksp for Bi(OH)3 if 1.1 x 10-8 moles of Bi(OH)3 dissolve in 1.0
liter of water to form a saturated solution.
![]()
Ans: Ksp = 4.0 x 10-31
![]()
![]()

16. Complete the following problem:
![]()
a. Calculate the solubility of BaSO4 in H2O. Ksp = 1.1 x 10-10.
![]()
Ans: 1 x 10-5 M
![]()
![]()
17. Complete the following problem:
![]()
a. Calculate the solubility of BaSO4 in 0.100 M Na2SO4.
![]()
Ans: 1.1 x 10-9 M
![]()
![]()
18. Complete the following problem;
![]()
a. A 50.0 mL sample of 0.0152 M Na2SO4 is added to 50.0 mL of 0.0125 M
Ca(NO3)2.
![]()
i) Should precipitation of CaSO4 occur?
![]()
ii) What % of the Ca2+ will precipitate?
![]()
Ans: 38 %
![]()
![]()
19a. Under what circumstances will the solubility of an ionic compound be dependent on the pH of the solution?
![]()
b. Give two examples of ionic compounds that are more soluble in acidic solutions
than basic solutions.
![]()
c. Give two examples of ionic compounds that are more soluble in basic solutions
than acidic solutions.
![]()
![]()
Problem Set #29
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
PS29.1. Calculate the pH of the following buffer solutions.
![]()
a) 0.250 M HC2H3O2 and 0.250 M NaC2H3O2
![]()
b) 0.00250 M HC2H3O2 and 0.00250 M NaC2H3O2
![]()
c) 0.470 M NH4Cl and 0.470 M NH3
![]()
![]()
d) 0.260 M HCN and 0.340 M NaCN
![]()
PS29.2. Specify the reagents (an acid and its conjugate base or a base and its
conjugate acid) and the concentration of each reagent needed to prepare
buffer solutions having the listed pH values.
NOTE: The optimum buffer solution is one with equal concentrations of the weak acid (weak base) and its conjugate base (conjugate acid). Under these conditions, the pH of the solution is equal to the pKa (pKb). So the best reagent for each of the solutions below is one whose pK is equal to the pH. Since the tables in the appendix list K values, each of the pH's must be converted to their corresponding [H+] and compared to an equilibrium constant in Appendix E1 or E2.
a) 4.74
![]()
b) 10.81
![]()
c) 5.23
![]()
![]()
PS29.3. Determine the pH of a buffer prepared by mixing 0.475 moles of HC2H3O2 and 0.525 moles of NaC2H3O2 in enough water to give 1.00 liter of solution.
![]()
Calculate the pH when
a) 0.0500 mol of HCl is added
![]()
b) 0.0500 mol of NaOH is added
![]()
![]()
c) 1 liter of water is added
![]()
d) 5.00 mL of a 1.00 M HNO3 solution is added
![]()
PS29.4. Calculate the pH change produced when 0.100 mol of solid NaOH is added
to each of the following buffer solutions.
![]()
a) 500 mL of 0.900 M HC2H3O2 and 0.900 M NaC2H3O2
![]()
b)500 mL of 0.550 M HC2H3O2 and 0.550 M NaC2H3O2
![]()
![]()
PS29.4.
c) 500 mL of 0.200 M HC2H3O2 and 0.800 M NaC2H3O2
![]()
d) 500 mL of 0.100 M HC2H3O2 and 0.900 M NaC2H3O2
![]()
PS29.5. Calculate the pH change produced when 0.100 mol of gaseous HCl is
added to each of the following buffer solutions.
![]()
a) 500 mL of 0.900 M NH3 and 0.900 M NH4Cl
![]()
![]()
b) 500 mL of 0.200 M NH3 and 0.800 M NH4Cl
![]()
![]()
c) 500 mL of 0.100 M NH3 and 0.900 M NH4Cl
![]()
PS29.6. Complete and balance the following reactions. Identify all products phases as
either (g)as, (l)iquid, (s)olid or (aq)ueous. If no reaction occurs, write NR.
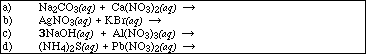
![]()
PS29.7. Calculate Ksp for the following salts using the information provided.
a) The concentration of CrO42-(aq) in a saturated solution of Ag2CrO4 is
6.50 x 10-5 M.
![]()
![]()
b) The solubility of AgBrO3 in water is 7.2 x 10-2 g/L.
![]()
c) A sample of a saturated solution of PbSO4 contains .0262 g/L of Pb2+.
![]()
PS29.8. Calculate the solubility of the following compounds in water. (Use a table of
solubility product constants in your text or some other reference book.)
![]()
a) BaCO3
![]()
![]()
b) AuCl
![]()
c) AuCl3
![]()
d) Cu3(PO4)2
![]()
PS29.9. Calculate the solubility of;
![]()
a) BaCO3 in 0.500 M Ba(NO3)2
![]()
![]()
b) PbCl2 in 0.0250 M CaCl2
![]()
c) Cu3(PO4)2 in 0.200 M Cu(NO3)2
![]()
![]()
PS29.10. A 45 mL sample of 0.015 M calcium chloride is added to 55 mL of 0.010 M sodium sulfate. Is a precipitate expected? Explain. (Your answer must include a calculation!)
![]()
![]()