 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Properties of Solutions
Chapter 12
Objectives
![]()
Following your study of this chapter, you should be able to
![]()
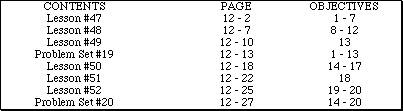
![]()
![]()

1. Define the following terms:
solution
![]()
solute
![]()
solvent
![]()
dissolution
![]()
concentration
![]()
![]()
2. Describe your observations of the following experiments performed by the instructor.
a) Sodium chloride added to water
![]()
b) Sodium metal added to water
![]()
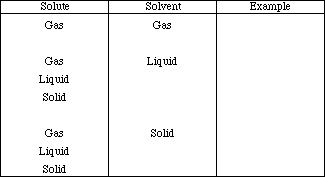
3. Complete the following table by providing physical examples of solute/solvent
combinations.
![]()
![]()
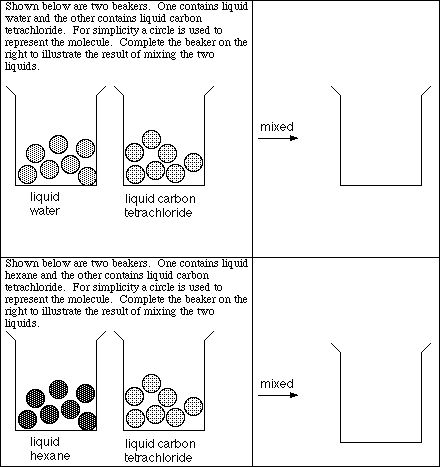
4. Based on the lecture demonstrations describe what happens when the following pairs of liquids are mixed together.
a) water and hexane
![]()
b) hexane and carbon tetrachloride
![]()
c) water and ethanol
![]()
5. List the set of rules which can be used to predict the polarity of a covalent
molecule. (Review: See Exercises 9 and 10 in Chapter 9 of the
Lectureguide)
![]()
![]()
6. Briefly describe each of the following types of intermolecular attractive forces. Sketch the orientations of molecules and/or ions involved in the following intermolecular attractive forces. Include at least one specific example where each attractive force is important. For each one, tell what causes the force and describe its strength relative to the others. (Review: See Exercises 10 and 11 in Chapter 11 of the Lectureguide.)
dipole-dipole forces
![]()
London dispersion forces
![]()
hydrogen-bonding forces
![]()
![]()
7. The three attractive interactions which are important in solution formation are; solute-solute interactions, solvent-solvent interactions, and solute-solvent interactions. Define each of these interactions and describe their importance in determining whether a particular solute-solvent pair will form a homogeneous mixture or a heterogeneous mixture.
![]()
![]()
![]()

8a. In terms of the attractive interaction discussed in exercise #6 explain how it is the formation of a solution can be exothermic or endothermic.
![]()
b. Describe the underlying thermodynamic property which favors the formation of a
solution. Explain why some combinations of chemicals do not form
homogeneous mixtures.
![]()
9a. Define the following terms;
solubility
![]()
unsaturated solution
![]()
saturated solution
![]()
supersaturated solution
![]()
![]()
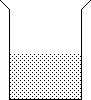
b. Given that the beaker to the left contains an aqueous solution of NaCl, describe a simple test to determine whether the solution is unsaturated, saturated or supersaturated.
What would you
expect to happen during the test if the solution
were unsaturated? saturated? supersaturated?
10a. Given the representations below, sketch the orientations of a chloride ion and a several water molecules and a sodium ion and several water molecules to illustrate the ion-dipole interaction.
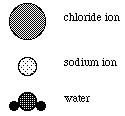
b) Briefly describe ion-dipole intermolecular attractive forces which occur when an ionic solid dissolves in water. Indicate what causes the attractive force and describe how the strength depends on the charge and the size of the ion.
![]()
![]()
11. Define the term lattice energy and explain its importance in the enthalpy of solution.
![]()
12. Explain how pressure, temperature and molar mass effect the solubility of a gas
in a liquid.
![]()
![]()

13a. Define the concentration terms;
weight percent
![]()
mole fraction
![]()
molarity
![]()
molality
![]()
![]()
b. Calculate the molality and mol fraction of HCl for a solution which is 37.1 % HCl by weight (mass).
![]()
Ans: 16.2 m
c. If the density of the solution described in b. is 1.18 g/mL, calculate the molarity of the solution.
![]()
Ans: 12.0 M
d. Calculate the weight percent NaCl in a solution which is 0.632 molal.
![]()
![]()
e. A solution of ethylene glycol, C2H4(OH)2, which is 6.77 molar has a density of 1.05 g/mL. Calculate the mole fraction of ethylene glycol in the solution.
![]()
Ans: 0.162
![]()
![]()
Problem Set #19
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
PS19.1. Predict whether in the following pairs (solute:solvent) a solution is formed.
In each case explain why or why not.
a)
HCl(g):H2O(l)
![]()
b)
CH3COOH(l):H2O(l)
![]()
c)
CH3OH(l):C7H8 (toluene)(l)
![]()
d)
C6H14(l):H2O(l)
![]()
e)
I2(s):C7H8 (toluene)(l)
![]()
f)
Br2(l):H2O(l)
![]()
g)
NaNO3(s):H2O(l)
![]()
h)
NaClO4(s):CCl4(l)
![]()
PS19.2. Predict whether the following compounds are soluble or insoluble in water.
a) Na2SO4(s)
b) C12H22O11(s)
c) CoCl2(s)
d) CH3OH(l)
e) C7H8(l)
f) HF(l)
g) NH3(g)
h) (CH3)2CO(l)
![]()
PS19.3. For those compounds in 19.2 that are soluble write the equation which
describes the compound's behavior when added to water.
![]()
![]()
PS19.4. Describe the attractive forces present when KI(s), NH3(g) and CH3CH2OH(l) dissolve in water. Prepare sketches depicting at the atomic level how each of these substances interact with water molecules.
![]()
![]()
PS19.5. If 52.6 g of AgNO3 are added to 684 mL of water, determine;
![]()
a) how many grams of AgNO3 (solute).
![]()
b) how many grams of H2O (solvent). (Density of pure water is 1.00 g/mL )
![]()
c) how many grams of solution.
![]()
d) how many moles of AgNO3.
![]()
e) how many moles of H2O.
![]()
f) how many moles of solution.
![]()
g) how many milliliters of solution, if the density is 1.106 g/mL.
![]()
h) the weight percent AgNO3.
![]()
i) the mole fraction of AgNO3.
![]()
j) the molality of the solution.
![]()
k) the molarity of the solution.
![]()
![]()
PS19.6. Given that an aqueous solution, which weighs 367 g, is 40.0 % (ethylene glycol) C2H6O2 by mass and has a density of 1.05 g/mL, determine;
![]()
a) the mole fraction of ethylene glycol.
![]()
b) the molality of the solution.
![]()
c) the molarity of the solution.
![]()
PS19.7. An aqueous solution of cesium chloride is 5.94 molal and has a density of
1.58 g/mL. Calculate the
a) weight percent cesium chloride.
![]()
b) mole fraction of cesium chloride.
![]()
c) molarity of the solution.
![]()
![]()
PS19.8. Describe how you would prepare;
a) 520 mL of a 0.760 M NaCl solution.
![]()
b) 37 g of a 15.2 % (by weight) solution of NaCl.
![]()
c) 210. g (grams of solution) of a 0.185 molal NaCl solution.
![]()
PS19.9. A 10.00 % (by weight) solution of K2SO4 in water was found to be 0.574 M
by titration at 25 ºC. Calculate
a) the molality of the solution
![]()
b) the density of the solution
![]()
![]()

14. Define the term colligative property and list those physical properties of a solution which can be classified as colligative properties.
![]()
15. Illustrate and explain how the presence of a nonvolatile solute affects the vapor
pressure of a liquid.
![]()
![]()
16a. Draw the vapor pressure versus temperature curve for water and label the important features. On the same diagram draw the vapor-pressure versus temperature curve for an aqueous solution containing a nonvolatile solute.
![]()
b. Write Raoult's law and define each term.
![]()
![]()
![]()
![]()
Ans: 23.5 mmHg
d. A solution was prepared by adding 20.0 g of urea to 125 g of water at 25 ºC, a temperature at which pure water has a vapor pressure of 23.76 mm of Hg. The observed vapor pressure of the solution was found to be 22.67 mm of Hg. Calculate the molecular weight of urea.
![]()
Ans: 59
![]()
![]()
![]()
e. Show the derivation of a mathematical relationship for the vapor pressure lowering (Pºsolvent - Psolution) of a liquid following the addition of a nonvolatile solute.
![]()
17. Draw the vapor pressure versus temperature curve for water and label the
important features. On the same diagram draw the vapor-pressure versus
temperature curve for an aqueous solution containing a nonvolatile solute.
Explain how the addition of a nonvolatile solute affects the freezing point and
boiling point of water.
![]()
![]()

18a. Write the general mathematical relation which describes the dependence of the freezing point or boiling point on the concentration of solution.
![]()
b. Calculate the freezing point and boiling point of a solution prepared by mixing 6.00 g of
C6H12O6 with 35.0 g of H2O.
![]()
Ans: Tfp = -1.77 ºC : Tbp = 100.486 ºC
![]()
![]()
c. A solution containing a nonelectrolyte dissolved in water has a boiling point of 100.305ºC. Calculate the freezing point of the same solution.
![]()
Ans: Tfp = -1.11 ºC
d. What is the molecular mass of nicotine if 5.04 grams of this compound changes the freezing point of 90.0 g of water by 0.647 ºC?
![]()
Ans: 161 g/mol
![]()
![]()
e. Calculate the freezing point and the boiling point of a saturated solution of Li2CO3. The solubility of lithium carbonate is 0.72 g per 100 g of water at 100 ºC.
![]()
Ans: Tfp = -0.544 ºC : Tbp = 100.149 ºC
![]()
![]()
f. 2.57 g of an ionic compound with the formula KX are dissolved in 120 g of water. The freezing point of the solution was lowered by 1.37 ºC. Determine the formula weight of X.
![]()
Ans: 19 g/mol
![]()
![]()

19a. Define the terms semipermeable membrane, osmosis and osmotic pressure.
![]()
b. Using a kinetic molecular model illustrate the movement of solvent molecules
across a semipermeable membrane which separates pure water from a solution of
sugar and water. Using this illustration explain what happens in reverse
osmosis.
![]()
20. Give three examples of colloids.
![]()
![]()
Problem Set #20
AP Chemistry by Satellite Name___________________________________
![]()
ALL work must be shown in all problems for full credit.
![]()
PS20.1. Calculate the vapor pressure for each of the following solutions at 25 ºC;
a) 16.8 g of urea (NH2)2CO dissolved in 108 g of water.
![]()
b) 8.54 g of MgCl2 dissolved in 108 g of water.
![]()
PS20.2. To what temperature (ºC) would a solution containing 150 g of glycerol,
C3H5(OH)3 (assume to be a nonvolatile solute), in 100 g of water have to be
heated to have a vapor pressure of 91.1 mmHg?
![]()
![]()
PS20.3. Determine the freezing point of a solution which is 0.50 molal urea (a nonelectrolyte). Determine the boiling point of this solution.
![]()
PS20.4. A solution containing a nonelectrolyte dissolved in water has a freezing point
of -1.62 ºC. Calculate the boiling point of the same solution.
![]()
PS20.5. Given the following data;

![]()
a) If each of the solutions is prepared by adding 1 mole of compound to 1 kg of water
why does each have a different DTf ?
![]()
b) Predict the ideal DTf for the above compounds.
![]()
![]()
PS20.5. (Continued)
![]()
b) Predict the ideal DTf for the above compounds.
![]()
c) Why does the ideal DTf differ from the experimental DTf?
![]()
d) Classify each compound as a strong, weak or nonelectrolyte.
![]()
PS20.6. Determine the ideal freezing point of a solution prepared by mixing 3.53 g of
MgCl2 in 430 g of water.
![]()
![]()
PS20.7. Calculate the "molecular formula" of solid sulfur if the freezing point of carbon tetrachloride is lowered by 0.28 ºC when 0.24 g of sulfur is added to 100 g of carbon tetrachloride. (Note: Kf(CCl4) = 29.8 ºC/m .)
![]()
PS20.8. When 7.20 g of HBr are added to 100 g of water, the freezing point of the
solution is lowered by 3.30 ºC. How can you explain this temperature
change? Is HBr a strong, weak or nonelectrolyte?
![]()
![]()
PS20.9. 5.76 g of an ionic compound with the formula K3X is dissolved in 350 g of water. The freezing point of the solution was lowered by 0.370 ºC. Determine the formula weight of X.
![]()
![]()