 Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index AP Chemistry by Satellite Lectureguide
Student Edition
Gas Laws
Chapter 10
Objectives
Following your study of this chapter, you should be able to
![]()
![]()
![]()

1a. Of the first 89 elements, list those which are gases in their standard state. Mark the gaseous elements on the periodic table.
![]()
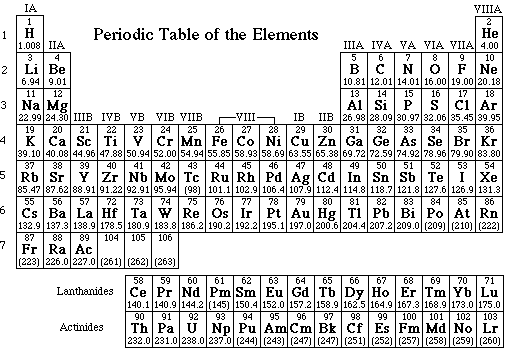
b) Of the first 89 elements list those which are liquids in their standard state:
![]()
![]()
1c. What phase are all remaining elements?
![]()
2. List all of the elements that are gases and identify their color.
3. Distinguish between the gas, liquid and solid phase by listing the unique properties of each that are not shared by the others.
![]()
4a. Define standard atmospheric pressure.
![]()
b) How is atmospheric pressure measured?
![]()
![]()
4c. Label the picture of the device used to measure atmospheric pressure and describe how it works.
5. Given a pressure of 1 atm, express the quantity in mmHg, Pascals (Pa) and kilopascals (kPa).
![]()
6a. Beginning with the equation F = ma, derive an equation relating atmospheric pressure (P)
to the density (r) of the liquid, the force of gravity (g) and the height of the liquid in a
barometer.
![]()
![]()

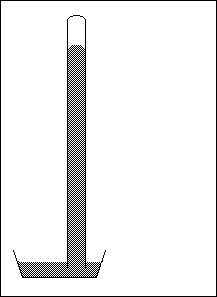
7. Sketch a picture of a manometer. Describe how it is constructed and how it is used to measure the pressure of a sample of gas. .
![]()
![]()
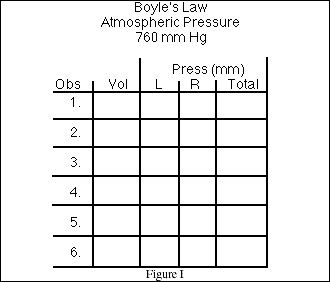
 8. Use the data table displayed in Figure I to record the data during the class experiment.
When the lecture is over, use the graph paper provided below to plot the data. Label the 'x'
and 'y' axis of the graph and clearly identify all data points. Select the range of values for
each axis to allow all data points to be displayed.
8. Use the data table displayed in Figure I to record the data during the class experiment.
When the lecture is over, use the graph paper provided below to plot the data. Label the 'x'
and 'y' axis of the graph and clearly identify all data points. Select the range of values for
each axis to allow all data points to be displayed.
![]()
![]()
![]()
8. (Continued)
Plot #1
Volume vs. Pressure Graph
![]()
Plot #2

Volume vs. 1/Pressure Graph
![]()
![]()

![]()
![]()
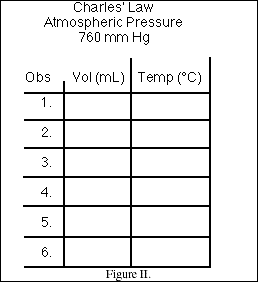
9. Use the data table displayed in Figure II to record the data during the class experiment. When the lecture is over, use the graph paper provided below to plot the data. Label the 'x' and 'y' axis of the graph and clearly identify all data points. Select the range of values for each axis to allow all data points to be displayed and the determination of the x-intercept (the point on the x-axis where y = 0).
![]()
![]()
![]()
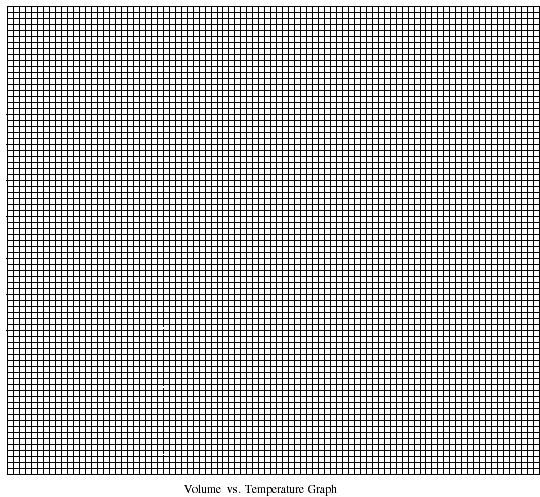
9. (Continued)
Plot #1
![]()
![]()

![]()
![]()

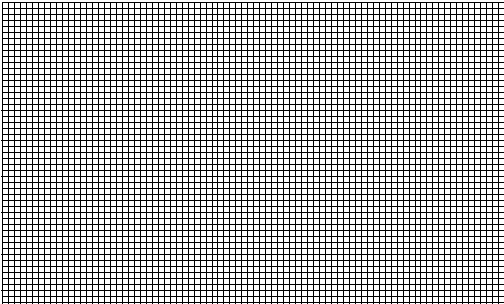
Using the graph paper below, extrapolate the data to determine the value of absolute zero. How does this value compare to value obtained in the Charles' Law experiment? (Note: Remember the minimum value of the temperature (x-axis) should be near -290 ºC.)
![]()
10. Describe the two independent experiments that yield a value for absolute zero.
![]()
![]()

11a. Write the ideal gas equation and define each of the variables.
![]()

![]()
![]()

![]()
![]()

13. Beginning with the mathematical form of the ideal gas law, rearrange the equation and show how Boyle's law, Charles' law and Avogadro's law can be obtained.
![]()
![]()
Problem Set #15
AP Chemistry by Satellite
![]()
ALL work must be shown in all problems for full credit.
![]()
PS15.1. The pressure of the mercury in the gas (vapor) phase above the liquid mercury in a
barometer is 2.0 x 10-3 mmHg. Calculate the pressure in units of atmospheres.
![]()
PS15.2. A sample of SF6 occupies a container of variable volume. If the the sample originally
occupies a volume of 50.0 mL at 755 mmHg, calculate the pressure which must be
exerted to lower the volume to 25.0 mL.
![]()
PS15.3. If the pressure of a sample of an ideal gas, initially at 1.25 atm, is tripled, by what
factor will the volume of the gas change?
![]()
PS15.4. A 545 mL sample of nitrogen gas initially at -220 ºC is heated to 100 ºC. Calculate
the new volume, assuming the pressure does not change.
![]()
PS15.5. Calculate the volume of 0.390 moles of an ideal gas at 750 mmHg and 23 ºC.
![]()
![]()
PS15.6. Calculate the density of SF6 at 1.00 atm and 0.00 ºC.
![]()
PS15.7. Calculate the volume of a sample of helium at -33.0 ºC and 1.23 atm if it occupies a
volume of 2.34 L at 54.5 ºC and 1026 mmHg.
![]()
PS15.8. A 0.751 mol sample of an ideal gas occupies a 10.0 liter flask at 27.0 ºC and 1.85 atm.
If 0.257 mol of the gas are removed from the container, calculate the new pressure.
(Assume the temperature remains constant.)
![]()
PS15.9. Which of the following contains the largest number of particles?
a) 5.00 g of He at 1.00 atm and 0 ºC
![]()
b) 225 g of Au
![]()
c) 34.5 L of an ideal gas at -5.0 ºC and 2000 mmHg
![]()
![]()
PS15.10. Which of the following samples has the greater mass?
a) 278 L of Ar at 25 ºC and 300 mmHg
![]()
b) 225 mL of CH4 at 300 ºC and 5.34 atm
![]()
c) 34.5 L of Cl2
![]()
![]()

15a. State Dalton's Law of partial pressures and explain how it is related to the ideal gas law equation.
![]()
![]()

![]()
![]()

16. State the principle postulates of the kinetic-molecular theory.
![]()
17. Explain in words the relationship between temperature of a sample of gas and the kinetic
energy of the particles of the gas.
![]()
![]()
18. In each of the following experiments, explain the macroscopic behavior of gases using kinetic-molecular theory.
![]()

![]()
![]()

![]()
![]()
![]()

![]()

![]()
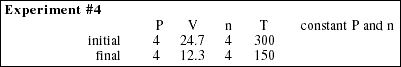
![]()
![]()
![]()

19a. Describe the difference between effusion and diffusion at the molecular level.
![]()

20a. Write the mathematical equation that describes the relative effusion or diffusion rates for ideal gases.
![]()
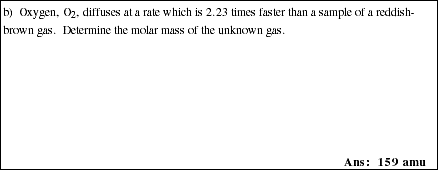
![]()
![]()
21a. List the conditions under which real gases deviate most from ideal behavior.
![]()
b) Discuss the postulates of the kinetic-molecular theory that are not true for real gases.
Explain how real gases behave in terms of these postulates.
![]()
c) Write the van der Waals equation for real gases and define the variables.
![]()
![]()
Problem Set #16
AP Chemistry by Satellite
ALL work must be shown in all problems for full credit.
![]()
PS16.1. The first laboratory preparation of O2, performed by Joseph Priestly, required the
heating of mercury (II) oxide,
![]()
![]()
What volume of O2 at 22.5 ºC and 753 mmHg will be produced when 15.3 g of HgO
are completely decomposed?
![]()
PS16.2. Hydrogen, H2, can be prepared by passing steam through a hollow air tube which
has been heated to high temperature. The reaction is,
![]()
![]()
![]()
Calculate the volume of H2 formed at 0.98 atm and a temperature of 450 ºC when
98.3 g of H2O are passed through an iron tube.
![]()
PS16.3. Calculate the volume of SO2(g) produced when 2.00 liters of O2 react with excess
sulfur at constant temperature and pressure.
![]()
![]()
PS16.4. A gaseous mixture contains 3.00 g of N2, 0.430 moles of Ar and 2.15 x 1023
molecules of CH4. If the total pressure of the mixture is 3.00 atm, calculate the partial pressure of each component.
![]()
PS16.5. Experimentally oxygen gas is frequently collected over water. When a sample of
oxygen, produced from a chemical reaction, is collected over water at 24 ºC, the total
pressure was 765 mmHg. Calculate the pressure due only to oxygen.
![]()
PS16.6. A sample of oxygen collected over water at 29 ºC exerts a total pressure of 759
mmHg. If the volume of the container is 125 mL calculate the mass of oxygen
present.
![]()
PS16.7. Can the speed of a molecule of a gas be doubled if the temperature of the gas sample
is held constant, yes or no? Briefly explain your answer.
![]()
![]()
PS16.8. Explain, in terms of the kinetic molecular model, why increasing the volume of a sample of an ideal gas decreases the pressure of the gas at constant temperature.
![]()
PS16.9. Rank the following gases in order of rate of diffusion. Explain your order.
![]()
Ne, CH4, SF6, CO2
![]()
PS16.10. Describe the two factors responsible for the deviation of real gases from ideal
behavior as assumed in the ideal gas equation.
![]()
![]()
Microcomputer software
Project SERAPHIM
Dr. John Moore
Department of Chemistry
University of Wisconsin
Madison, WI 53706
AP401 BOYLE
CHARLES
GAS LAWS
BALLOON
BOYLE'S LAW SIMULATION
AP402 GAS LAW
BALLOON
GAS LAW 7
AP403 DALTON
![]()
Introduction to General Chemistry by Stan Smith, Ruth Chabay and Elizabeth Kean
Drill-and-practice software
$500 (10-disk set)
Falcon Software
P.O Box 200
Wentworth, NH 03282
1-603-764-5788
Diskette #7 Ideal Gases
![]()
Computer Aided Instruction for General Chemistry by William Butler & Raymond Hough
Drill-and-practice software
$40 (4-disk set)
John Wiley & Sons, Inc.
605 3rd Avenue
New York, NY 10158
(this software may not be available)
Diskette #2 Gases
![]()
Simulation instruction for Introductory Chemistry by John I. Gelder and R. E. Snelling
$50
High Technology Software Products, Inc.
P.O. Box 60406
Oklahoma City, OK 73146
CHEM LAB SIMULATIONS #2 Ideal Gas Law
![]()
![]()