 Go to Main Index
Go to Main Index Go to Main Index
Go to Main IndexFrom 5th Ed. of Brown, LeMay & Bursten
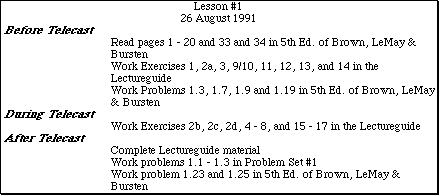
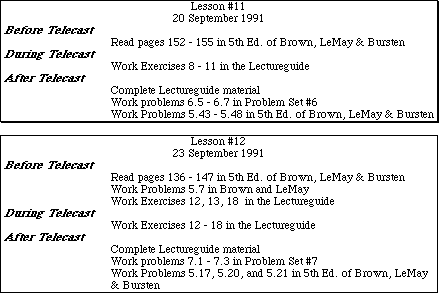
Listed on these pages you will find information boxes from the beginning of each lesson in the AP Chemistry by Satellite Lectureguide. The reading and pre- and post- telecast problem assignments from the 4th edition of the text by Brown and LeMay have been updated to the new 5th edition of the text by Brown, LeMay and Bursten. In most cases the reading material listed is virtually identical to that in the 4th edition. The problems sited from the 5th edition are either identical or very similar to those assigned from the 4th edition. Throughout the Lectureguide lessons, problems in the text similar to certain exercises have been referenced. These problems have not been updated to the new edition of the text.
In addition, some of the Problem Sets in the Lectureguide contain problem assignments from 4th edition of the text. A list of the correct problem numbers from the 5th edition has been included. In cases where an assigned problem no longer exists in the new text, the problem has been reproduced.
If you encounter any errors or omissions while using this document, please contact the AP Chemistry by Satellite office at 800-633-1176.
![]()
![]()


![]()

![]()


![]()

![]()

![]()


![]()

![]()

![]()


![]()

![]()

![]()


![]()

![]()

![]()


![]()

![]()

![]()


![]()

![]()

![]()


![]()

![]()

![]()


![]()

![]()

![]()


![]()

![]()

![]()

Problem Set
Problems from the text
PS2.5. Problem 3.18 in Brown and LeMay
Problem 3.18 in 5th Ed. of Brown, LeMay and Bursten
![]()
PS2.8. Problem 2.49 in Brown and LeMay.
"Many familiar substances have common, unsystematic names. In each of the
following cases. give the correct systematic name: (a) saltpeter (KNO3); (b)
soda ash (Na2CO3); (c) lime (CaO); (d) baking soda (NaHCO3); (e) lye
(NaOH); (f) muriatic acid (HCl); (g) milk of magnesia (Mg(OH)2), (h) dry ice
(CO2); ammonia (NH3)."
PS8.1. Exercise 5.8 in Brown & LeMay
Exercise 7.22 in 5th Ed. of Brown, LeMay & Bursten
PS8.2. Exercise 5.10 in Brown & LeMay
"(a) Arrange the following series of elements in order of increasing metallic
character: Si, Sn, C, Ge, Pb. (b) Arrange the following series in order of
increasing nonmetallic character: As, P, Bi, Sb, N."
PS8.3. Exercise 5.11 in Brown & LeMay
"Write the chemical formula of the compound formed by (a) magnesium and
iodine; (b) gallium and oxygen; (c) barium and bromine."
PS8.4. Exercise 5.13 in Brown & LeMay
Exercise 7.25 in 5th Ed. of Brown, LeMay & Bursten
PS8.5. Exercise 5.23 in Brown & LeMay
Exercise 4.43 in 5th Ed. of Brown, LeMay & Bursten
PS8.6. Exercise 5.25 in Brown & LeMay
Exercise 4.45 in 5th Ed. of Brown, LeMay & Bursten
PS8.7. Exercise 5.28 in Brown & LeMay
Exercise 4.48 (a) and (b) in 5th Ed. of Brown, LeMay & Bursten and
"(c) Predict whether the following reaction will occur:
![]()
![]()
![]()
PS8.8. Exercise 5.17 in Brown & LeMay
Exercise 7.29 in 5th Ed. of Brown, LeMay & Bursten
PS8.9. Exercise 5.32 in Brown & LeMay
"What is the charge of each of the following ions: (a) selenide; (b) astinide;
(c) phosphide; (d) cesium?"
PS8.10. Exercise 5.34 in Brown & LeMay
"From the following elements - oxygen, sulfur, lithium, silver, iodine,
germanium, aluminum - pick the one that best fits each of the following
descriptions; (a) an active metal that forms a cation with a 1+ charge; (b) a
grayish-black solid that readily forms a purple vapor; (c) a metal that dissolves
in NaOH solution; (d) a metalloid; (e) a yellow nonmetal."
PS14.4. Problem 9.27 in Brown and LeMay.
Problem 9.32 in 5th Ed. of Brown, LeMay & Bursten