Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam III
April 11, 1991
![]()
INSTRUCTIONS:
1. This examination consists of a total of 12 different pages. The last 4 pages include important mathematical equations and constants, a solubility table, a periodic table, and tables of dissociation constants, solubility constants and thermodynamic values. All work should be done in this booklet. You may carefully remove the last 4 pages of the examination.
2. PRINT your name, high school, teaching partner's name and today's date now in the space at the top of this sheet. DO NOT SEPARATE THE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. You do not have to show your work for the multiple choice or short answer questions.
4. No credit will be awarded if your work is not shown in problem 2 - 6, 7a, 7b, 7d, and 7e. Please circle your final answer!
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax
![]()

APCBS Exam III 2
![]()
(9) 1. Complete and balance the following reactions. Identify all product's phases as either (g)as, (l)iquid,
(s)olid or (aq)ueous. Products which are soluble ionic compounds must be written as ions. If no reaction
occurs, write NR.
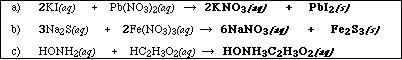
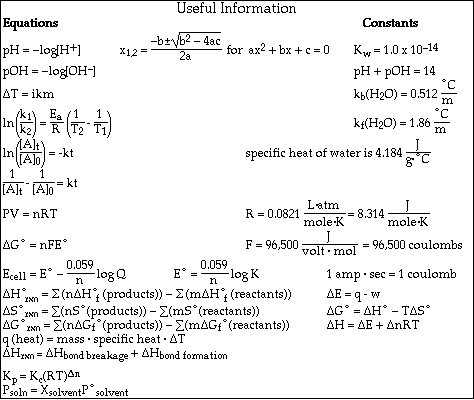
(8) 2. Calculate the pH of a solution formed after mixing 25.0 mL of 0.500 M HCl and 40.0 mL of 0.350 M KOH.
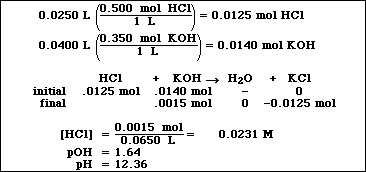
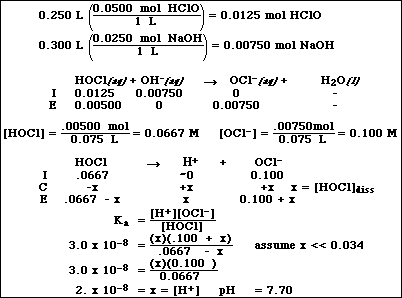
(8) 3. Calculate the pH of a solution formed after mixing 50.0 mL of 0.250 M HClO and 25.0 mL of 0.300 M NaOH.
APCBS Exam III 3
![]()
(16)4a. Calculate the pH of a solution which is 0.750 M HC4H7O2 (butyric acid) and 0.650 M NaC4H7O2.
![]()
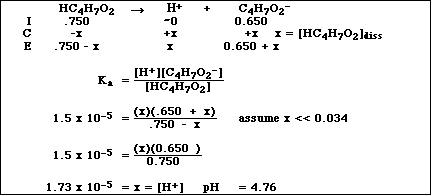
![]()
b. Calculate the pH after the addition of 0.010 mol of KOH to a 400 mL sample of the buffer in Problem
#4a.
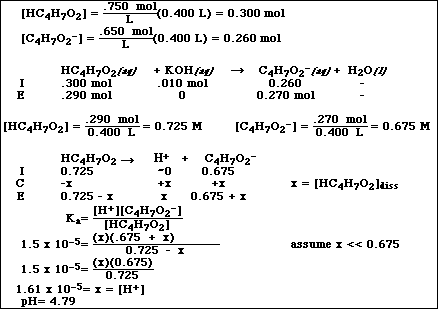
APCBS Exam III 4
![]()
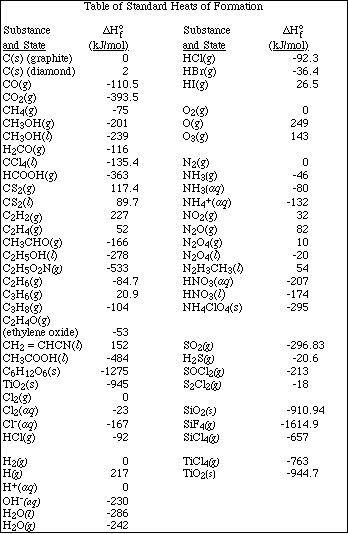
(7) 5. Calculate the solubility, in mol/L, of CaF2.
![]()
Write the equilibrium equation-
![]()
and the equilibrium expression-
![]()
Ksp = 3.9 x 10-11 = [Ca2+][F-] 2
![]()
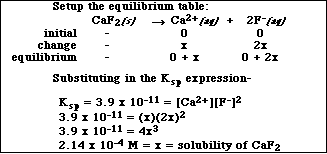
![]()
(7) 6. If 50.0 mL of a saturated solution of CaF2 (see Problem 5) is mixed with 50.0 mL of 0.00350 M
solution of Na2CO3, will a precipitate of CaCO3 form? (Note: You must show calculations to support
your answer.)
![]()
From Problem 5 the concentration of Ca2+ in the solution is 2.14 x 10-4 M. So after
mixing with 50.0 mL of 0.00350 M CO32-, the new concentrations are,
![]()
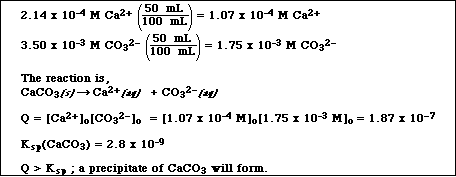
APCBS Exam III 5
![]()
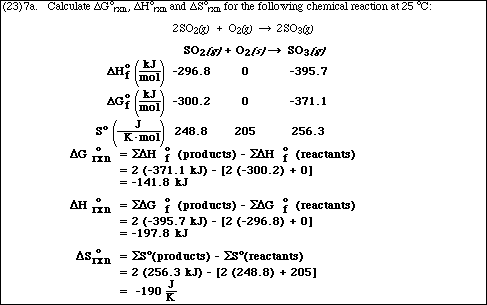
b.What would be the values of each of these three functions at 500 ºC? (Note: State any assumptions)

![]()
c. Write the equilibrium constant expression, Kp, for the reaction
![]()
![]()
APCBS Exam III 6
![]()
d.Calculate the value of Kp at 25 ºC and at 500 ºC.

![]()
7.e. If a 1.00 liter vessel contains a partial pressure of each gas equal to 3.00 x 10-4 atm, will the reaction
proceed spontaneously in the forward direction at 25 ºC? 500 ºC? (Note: Your answer must include
calculations and a brief written explanation to receive credit.)
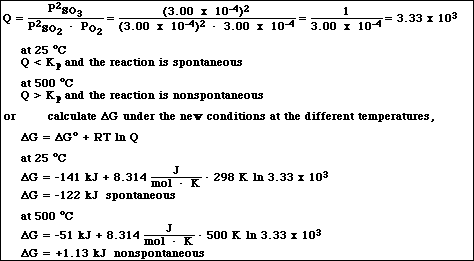
APCBS Exam III 7
![]()
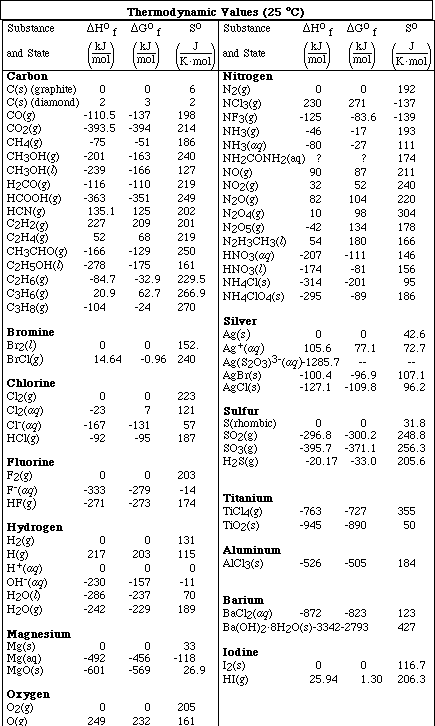
(8) 8. Given the reactions
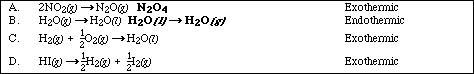
a. Predict the sign of DSºrxn for each of the four reactions. Explain your reasoning for full credit.
A. DSº is - since there is a reduction in the number of moles of gas
B. DSº is - converting gas to a liquid
(B.) DSº is + converting liquid to a gas
C. DSº is - converting gas to a liquid and a reduction in the number of moles of
particles
D. DSº is + converting a pure substance to a mixture OR DSº is -, the atomic forms,
H2 and I2 are more orderly than the molecular form HI. Either answer with
explaination is accecpable.
b. Which reactions, if any, are spontaneous at high temperatures but not at low temperatures?
none of the reactions (DHº and DSº must both be +.)
c. Which reactions, if any, are spontaneous at low temperatures but not at high temperatures?
A and C (DHº and DSº must both be -.)
d. Which reactions, if any, are spontaneous at all temperatures?
D (DHº must be - and DSº must both be +
APCBS Exam III 8
Multiple Choice: (14 points)
Print the letter (A, B, C, D, E) which corresponds to the answer selected.
9. C ![]() 10. D
10. D ![]() 11. C
11. C ![]() 12. B
12. B
13. B ![]() 14. E
14. E ![]() 15. C
15. C
![]()
ONLY THE ANSWERS IN THE AREA ABOVE WILL BE GRADED. Select the most correct answer
for each question. Each question is worth 2 points.
Use the titration curve below to answer questions 9 - 11.

9. Which point indicates the region where the solution behaves as a buffer?
A) A
B) B
C) C
D) D
E) E
10. Which point indicates the equivalence point of the titration?
A) A
B) B
C) C
D) D
E) E
11. The titration curve best describes a titration between
A) a strong acid and a strong base
B) a strong acid and a weak base
C) a weak acid and a strong base
D) a weak acid and a weak base
APCBS Exam III 9
![]()
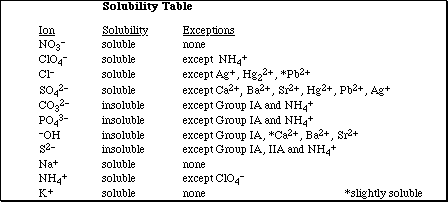
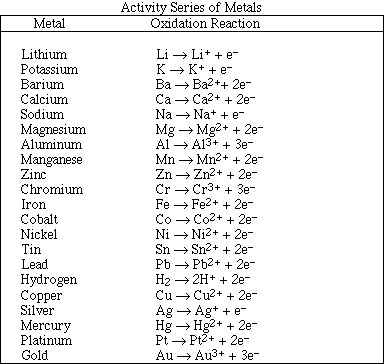
12. If 100 mL samples of saturated solutions of each of the lead salts listed below were prepared, which
would contain the highest concentration of Pb2+(aq)?
A) PbCO3 (Ksp = 7.4 x 10-14)
B) PbCl2 (Ksp = 1.6 x 10-5)
C) PbCrO4 (Ksp = 2.8 x 10-13)
D) PbF2 (Ksp = 2.7 x 10-8)
E) PbS (Ksp = 8.0 x 10-28)
![]()
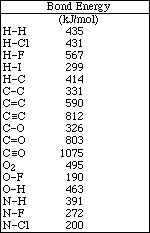
13. Calculate the magnitude of the equilibrium constant for the following reaction
![]()
A) 1.0 x 1014
B) 5.6 x 104
C) 1.8 x 109
D) 1.0 x 107
14. According to the second law of thermodynamics
A) the entropy change of the system must be positive for all spontaneous reactions.
B) the entropy change of the surroundings must be greater than the entropy change of the system for a
spontaneous process.
C) the entropy of the universe is constant, and only changes in the entropy of the system and
surroundings can be measured.
D) the entropy of a pure substance at absolute zero is 0 J/mol·K.
E) the entropy of the universe increases for all spontaneous processes.
15. If we could perform chemical reactions at absolute zero of temperature,
A) the free energy of the reaction would be equal to the entropy change.
B) the free energy would be negative for all reactions.
C) the free energy would be equal to the enthalpy change.
D) the free energy would be equal to zero for all reactions.
E) the free energy would be positive for all reactions.