Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam III
November 12, 1991
![]()
INSTRUCTIONS:
1. This examination consists of a total of 11 different pages. The last three pages include a periodic table and some useful information, a table of standard bond energies and an activity series. All work should be done in this booklet.
2. PRINT your name, high school, teaching partner's name and the date now in the space at the top of this sheet. DO NOT SEPARATE THESE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. You do not have to show your work for the multiple choice or short answer questions.
4. No credit will be awarded if your work is not shown in problems 7 or 8.
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()
APCBS Exam III KEY PAGE 2
![]()
(9) 1. Write the chemical formula(s) of the product(s) and balance any three of the following five reactions.
Identify all products phases as either (g)as, (l)iquid, (s)olid or (aq)ueous.
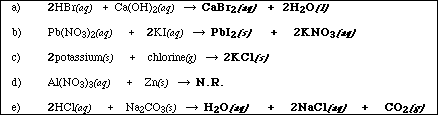
(14)2a. Consider the energy levels of a hypothetical atom,

![]()
(2)b. Draw an arrow on your diagram showing the transition of an electron from E4 to E1.
![]()
(2)c. Is energy absorbed or emitted when this transition occurs?
Energy is emitted in this transition.
(3)d. Calculate the energy of the photon of light absorbed or emitted.
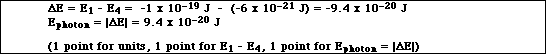
(4)e. Calculate the frequency of the photon of light absorbed or emitted.
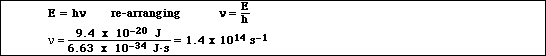
(1 point for units, 1 point for math, 2 points for the correct equation)
APCBS Exam III KEY PAGE 3
![]()
(12) 3. Answer Option 1 and any one of the remaining three options on this page.
Option 1: Which has the smaller atomic radius, Cl or Si? Explain your answer in terms of effective nuclear charge and shielding effects.
Cl has a smaller atomic radius compared to Si. Both elements have their outer electrons in the 3p orbitals. Si has a nuclear charge of +14 and Cl has a nuclear charge of +17. Since the electrons in the 3p orbitals do not shield each other fromn the charge on the nucleus, the 3p electrons in Cl "feel" a greater effective nuclear charge compared to the electrons in Si. The result is the 3p electrons in Cl are pulled closer to the nucleus and the atomic radius is smaller compared to Si.
(2 points for Cl smaller than Si, 2 points for shielding and 2 points for effective nuclear charge. In explaining effective nuclear charge 1 point for the fact that it is higher for Cl compared to Si and 1 point for the connection that the higher e.n.c. results in a greater attraction and a smaller radius.)
Option 2: Briefly explain what the term degeneracy means when used to describe features of the energy level diagram for a multi-electron atom.
Degeneracy means equal in energy. In a multi-electron atom, orbitals in the same subshell are degenerate. For example the three orbitals in the p subshell are all degenerate in energy.
(3 points for degeneracy and 3 points for sublevel idea.)
Option 3: Define electronegativity and state the overall trend observed going down a group and across a period in the periodic table.
![]()
Electronegativity is a measure of the attraction an atom has for the electrons it
shares in a chemical bond. The electronegativity decreases going down a group
and it increases going across a period.
(2 points for the correct definition, 2 points for the group trend, 2 points for the period trend.)
Option 4: Briefly describe the difference between an orbit and an orbital as it relates to an electron in a hydrogen atom.
An orbit is a circular path around the nucleus. According to Bohr's model, only orbits with specific radii are allowed. Orbits are specified by a single quantum number. An orbital is a volume of space specified by 3 quantum numbers where the probability of finding the electron is greatest.
(3 points for the orbit explanation and 3 points for the orbital.)
APCBS Exam III KEY PAGE 4
![]()
4. Short answer.
(5) a) Which of the following has the highest lattice energy
MgF2, KF, NaF, AlF3, RbF
![]()
Briefly, explain your choice.

(4) b) Write the possible set of quantum numbers for a valence electron in an antimony (Sb) atom in its ground state.
![]()
[Kr]5s24d105p3 : the valence electrons are in the 5s or the 5p subshell.
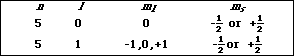
![]()
(4) c) Given the five orbital diagrams labeled A, B, C, D, and E.
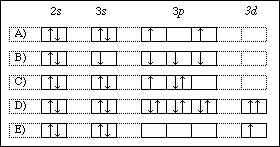
From the orbital diagrams select an example which demonstrates
![]()
i). a violation of Hund's rule C
ii). a violation of the Pauli exclusion principle D
iii). a ground state orbital diagram A
iv). an excited state orbital diagram E or B
v). a violation of the Aufbau principle B or E
APCBS Exam III KEY PAGE 5
![]()
(15) 5. Draw a Lewis electron-dot structure for each of the covalent molecules below. Include all resonance
structures if they are needed to adequately represent the bonding in the molecule.
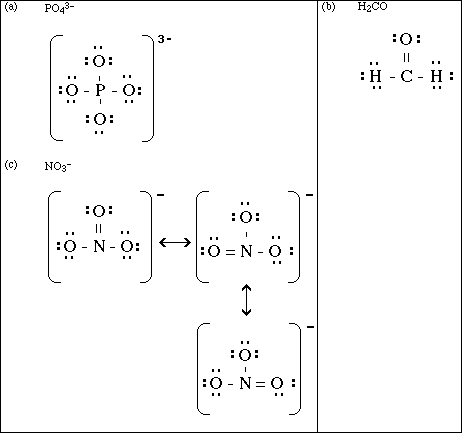
![]()
(8) 6. Write the electron configuration for each of the following.
a) Se2-
[Ar] 4s23d104p6 or 1s22s22p63s23p64s23d104p6
b) Mn
[Ar] 4s23d5 or 1s22s22p63s23p64s23d5
c) Ir
[Xe] 6s24f145d7 or 1s22s22p63s23p64s23d104p65s24d105p66s24f145d7
APCBS Exam III KEY PAGE 6
![]()
(10) Solve ONE of the two problems on this page. (A second problem will not be scored.)
7. An APCBS student went into the laboratory to run Experiment 5: Heat of Neutralization. After
calibrating the thermistor, the student began to measure the heat capacity of the calorimeter. 50.0 mL of
56.3 ºC water was added to 50.0 mL of 24.0 ºC water in the calorimenter. The maximum temperature
of the mixture was 42.1 ºC. The density of water is 1.00 g/mL and the specific heat of water is 4.184
J/g ºC.
A) Calculate the heat capacity of the calorimeter.
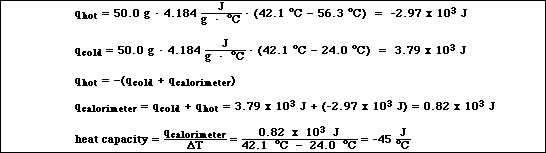
![]()
B) Explain why the student should not be satisified with the value of the heat capacity of the calorimeter
obtained in Part A.
The heat capacity is negative, so when heat is absorbed, it gets colder (lower temperature). This is the opposite of what is expected, so the student has made an error in the experiment.
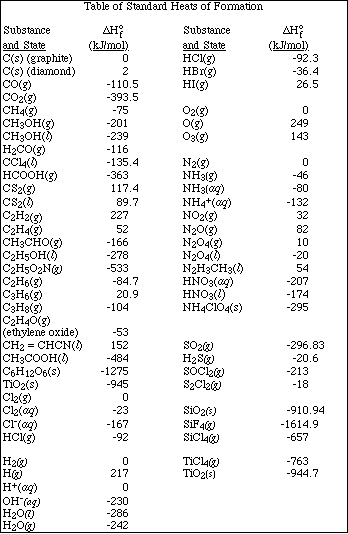
8. Using bond energies calculate DHº for the reaction
![]()

(Deduct 2 points for a math error or if units are missing in the answer. Deduct 4 points for the first error in the number of bonds that are broken or formed or if the wrong value of the bond energy is read from the table.)
APCBS Exam III KEY PAGE 7
![]()
Multiple Choice:
Print the letter (A, B, C, D, E) which corresponds to the answer selected.
9. A ![]() 10. D
10. D ![]() 11. A
11. A ![]() 12. C
12. C ![]() 13. A
13. A
14. D ![]() 15. D
15. D ![]() 16. B
16. B ![]() 17. E
17. E
ONLY THE ANSWERS IN THE AREA ABOVE WILL BE GRADED. Select the most correct answer for each question. Each question is worth 2 points.
9. Which of the following elements has the largest ionization energy?
A) O
B) B
C) I
D) Cs
E) S
![]()
10. Which of the following is isoelectronic with Ba2+?
A) Ca2+
B) La2+
C) O2-
D) I-
E) Rn
11. Which of the following has the smallest radius?
A) N
B) B
C) Al
D) Be
E) C
12. Which of the following groups contains compounds that do not obey the octet rule
A) NH3, PH3, SO3
B) H2O, CH2Cl2, CO2
C) BF3, SF4, ICl3-
D) NO2-, SO32-, SCN-
E) HOCl, Cl2CO, N2H4
APCBS Exam III KEY PAGE 8
![]()
13. A possible set of quantum numbers for the last electron added to complete the ground state electron
configuration for a neutral zinc atom is,
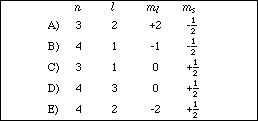
14. In which of the following substances is nitrogen in the most positive oxidation state?
A) NO
B) NO2
C) NO2-
D) N2O5
E) NH4+
15. An atom of the element X has the electron configuration _____ 1s22s22p63s23p3
The compound most likely to form with Br is,
A) XBr
B) XBr2
C) X2Br3
D) XBr3
E) X3Br2
16. What is the electron configuration of a Co3+ ion?
A) [Ar]4d6
B) [Ar]3d6
C) [Ar]4s23d10
D) [Ar]4s23d4
E) [Ar]4s23d7
17. Which of the following is isoelectronic with the nitrate ion?
A) NF3
B) H3O+
C) SO32-
D) NO2-
E) CO32-