Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam II
March 10, 1992
![]()
INSTRUCTIONS:
1. This examination consists of a total of 9 different pages. The last two pages include important mathematical equations and constants, a periodic table and tables of acid and base dissociation constants. All work should be done in this booklet.
2. PRINT your name, high school, teaching partner's name and today's date now in the space at the top of this sheet. DO NOT SEPARATE THE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. You do not have to show your work for the multiple choice or short answer questions. Clearly state any assumptions used when solving equilibrium problems.
4. No credit will be awarded if your work is not shown in problem 1, 2, 8 and 9. Please circle your final answer!
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()

APCBS Exam II PAGE 2
![]()
(30) 1. Calculate the magnitude of the equilibrium constant for any three of the four following cases;
a) Given the reactions
![]()
Express the equilibrium constant, K3, for the reaction below, in terms of K1 and K2
![]()
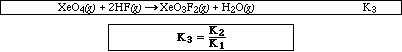
![]()
b) At a given temperature a gas mixture has reached equilibrium with the following partial pressures:
PH2 = 0.12 atm, PN2 = 0.70 atm, PNH3 = 0.84 atm
Calculate the equilibrium constant, Kp, for the reaction,
![]()
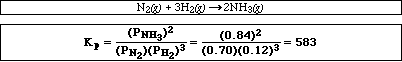
![]()
c) At 298 K 1.00 mol of OF2 is introduced into a 1.00 liter container and allowed to decompose according
to the reaction,
![]()
![]()
At equilibrium the concentration of OF2 is 0.785 M. Calculate Kc for the reaction.
![]()
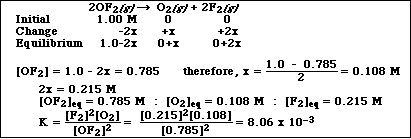
APCBS Exam II PAGE 3
![]()
d) Given the reaction
![]()
![]()
A container initially has a sample SO2Cl2 at 1.50 atm with no other species present. When equilibrium
is reached the final pressure of the mixture is 2.54 atm. Calculate Kp for the reaction.
![]()
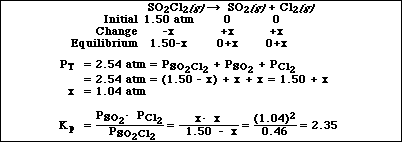
(10) 2. Answer ONE of the following questions;
![]()
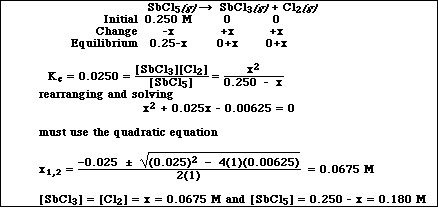
a) The equilibrium constant, Kc, at 448 ºC for the decomposition of antimony pentachloride,
![]()
![]()
![]()
is 0.0250. If 0.250 mol of SbCl5 are introduced into a 1.00 liter container at 448 ºC, calculate the
concentrations of all species when the reaction reaches equilibrium.
APCBS Exam II PAGE 4
![]()
b) The magnitude of the equilibrium constant for the reaction,
![]()
is 54.7 at a certain temperature. If the initial concentration of BrCl is 0.750 M and no other species are present, calculate the concentrations of all species when the reaction reaches equilibrium.

(12) 3a. Below is a schematic diagram of a simple spectrometer.
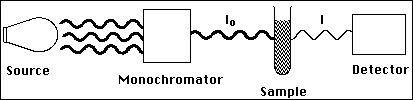
Briefly explain what transmittance (T) is, and how a spectrometer measures the transmittance of the sample.
![]()
Trnasmittance is a measure of the amount of light which passes through the sample.
The detector measures the light intensity Io with no sample in the beam. This value is
recorded by the instrument. With the sample in place, I is measured and compared to
Io. T is calculated as the ratio of I and Io.
APCBS Exam II PAGE 5
![]()
b) Complete the standard plot below by drawing the best line through the data points. Use the plot to
answer the questions in parts c) - e).
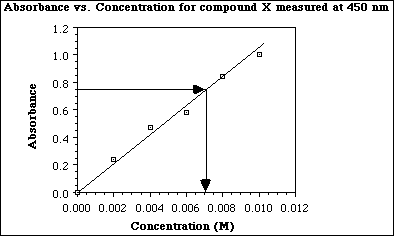
c) What is the concentration of compound X in a solution having an absorbance of 0.75? Clearly explain in words or show on the plot how the answer was obtained.
[X] = 0.0072 M
The concentration of X is determioned by drawing a line parallel to the x-axis from the absorbance of 0.75 to the best straight line through the data. Another line is drawn, parallel to the y-axis to the x-axis to determine the [X].
![]()
d) If lmax for the solution of compound X is 450 nm (blue light), what color would you expect the solution
to be?
Yellow or orange
e) What is the name of the equation of the curve (or the relationship illustrated) in the plot above?
Beer's Law
(4) 4. Write the equation for the autoionization of water. What is the concentration of H+ and OH- in a sample of pure water?
![]()
APCBS Exam II PAGE 6
![]()
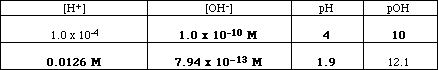
(6) 5. Fill in the table below.
(8) 6. Consider the reaction:
![]()
Will the equilibrium shift to the left, to the right, or not change under each of the following conditions?
![]()
a) [A2] = [B2] = 1 M, [AB] = 3 M
from left to right
b) Temperature changes from 25 ºC to 40 ºC
from left to right
c) Pressure doubles
no change
d) AB is removed from the system
from left to right
(4) 7. Calculate the pH of a 2.5 M NaOH solution.
Since NaOH is a strong base the [OH-] = 2.5 M
pOH = -log[OH-] = - log(2.5) = -0.400
pH + pOH = 14 therefore, pH = 14 - pOH = 14 - (-0.400) = 14.4
APCBS Exam II PAGE 7
![]()
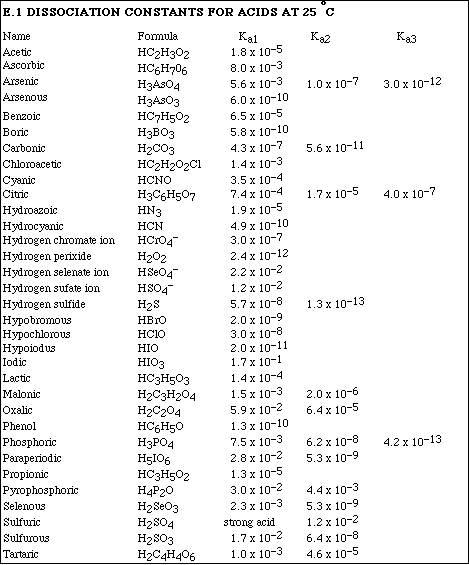
(10) 8. Calculate the pH of 0.400 M solution of ascorbic acid, HC6H7O6.
![]()
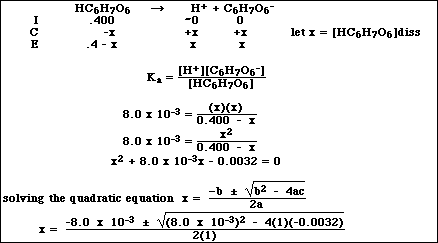
Use only the positive root
x = 0.0527 M = [H+ ]
pH = 1.28
![]()
(10) 9. Pyridine, C5H5N, behaves as a base in aqueous solution. A solution of pyridine with an initial
concentration of 0.750 M has a pH of 9.53. Calculate Kb for this compound.
![]()
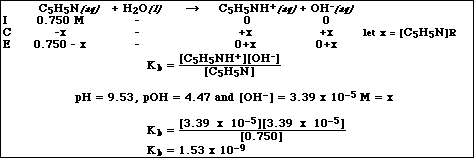
APCBS Exam II PAGE 8
![]()
Multiple Choice: (20 points)
Print the letter (A, B, C, D, E) which corresponds to the answer selected.
10. E ![]() 11. B
11. B![]() 12. D
12. D
ONLY THE ANSWERS IN THE AREA ABOVE WILL BE GRADED. Select the most correct answer for each question. Each question is worth 2 points.
![]()
10. Which of the following statements is true for chemical reactions?
A. If the rate constant is very large, the equilibrium constant is also very large.
B. Very slow reactions have small equilibrium constants.
C. Any factor that increases the rate constant will also increase the equilibrium constant.
D. If we know the value of the equilibrium constant we can determine the rate constant without further
experimentation.
E. We must determine the value of both the rate constant and the equilibrium constant in order to
understand and control the reaction.
11. For an exothermic reaction an increase in temperature will
![]()
A. increase both the rate and the equilibrium constant.
B. increase the rate and decrease the equilibrium constant.
C. decrease the rate and increase the equilibrium constant.
D. decrease both the rate and the equilibrium constant.
E. increase the rate and have no affect on the equilibrium constant.
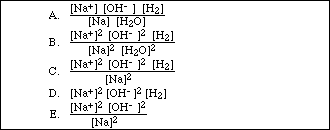
12. Consider the reaction:
![]()
Which of the following is the correct equilibrium constant expression for the reaction?