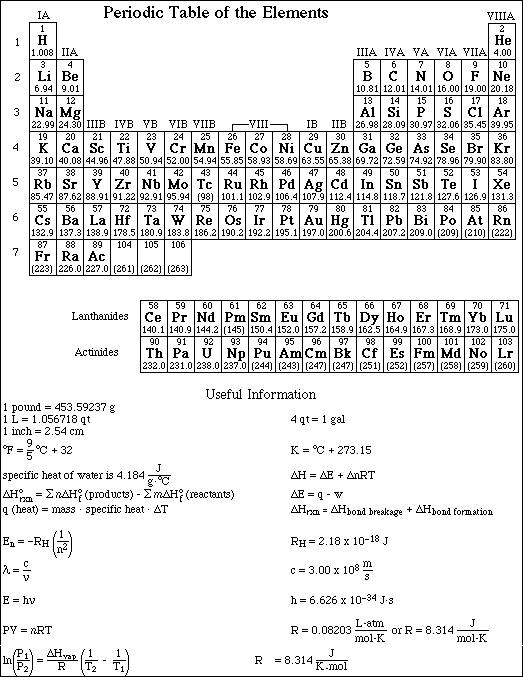Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam II
October 11, 1990
![]()
INSTRUCTIONS:
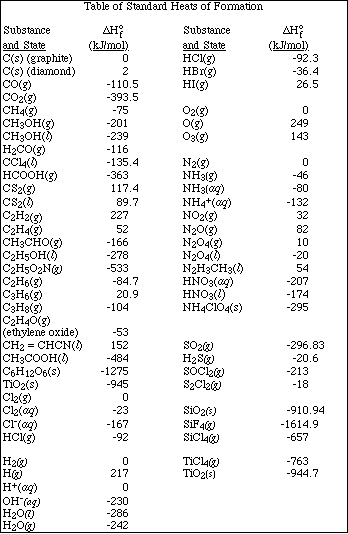
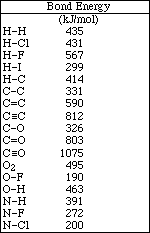
1. This examination consists of a total of 11 different pages. The last three pages include a periodic table and some useful information, a table of standard heats of formation and an activity series. All work should be done in this booklet.
2. PRINT your name, high school, teaching partner's name and the date now in the space at the top of this sheet. DO NOT SEPARATE THESE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. You do not have to show your work for the multiple choice or short answer questions.
4. No credit will be awarded if your work is not shown in problems 2 - 7.
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()
APCBS Exam II PAGE 2
![]()
(9) 1. Write the chemical formula(s) of the product(s) and balance the following reactions which were
demonstrated in lecture. Identify all products phases as either (g)as, (l)iquid, (s)olid or (aq)ueous.
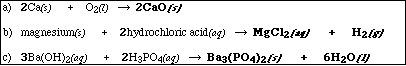
![]()
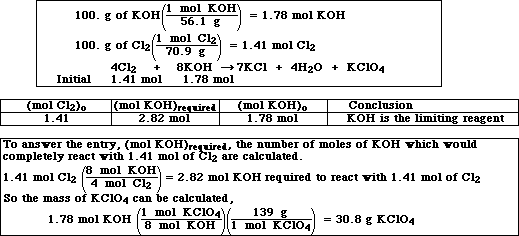
(9) 2. The overall equation for the preparation of potassium perchlorate, a powerful explosive, is,
![]()
![]()
Calculate the mass of KClO4 which can be produced when 100. g of KOH are reacted with 100. g of
Cl2. (Molar mass of Cl2 is 70.9 g and KOH is 56.1 g.)
Very similar to Exercise 17d in Chapter 3:
![]()
(8) 3. Calculate the DHºrxn for the combustion of glycine, an important amino acid,
![]()

APCBS Exam II PAGE 3
![]()
(10) 4. Calculate the concentration of a phosphoric acid, H3PO4, solution if 24.00 mL is neutralized by 12.00
mL of a 0.600 M NaOH solution.
Very similar to Exercise 22d in Chapter 3:
Important neutraization reaction,
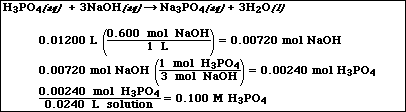
![]()
(8) 5. Using the following standard enthalpy of reaction data,

to calculate DHº for the following reaction
![]()
Very similar to Exercise 19c in Chapter 4:
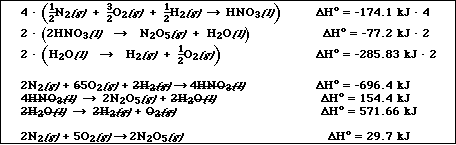
APCBS Exam II PAGE 4
![]()
(8) Solve ONE of the two problems in this part. (A second problem will not be scored.) Show all
important calculations and provide a brief explanation of how the solution is prepared.
6. Describe how you would prepare the following solution,
![]()
a) 250.00 mL of 0.450 M NaNO3 from NaNO3(s) and water
![]()
Very similar to Exercise 21e in Chapter 3:
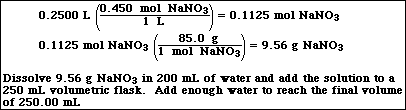
![]()
b) 1200 mL of 2.50 M H2SO4 from 9.00 M H2SO4
![]()
Very similar to Exercise 21f in Chapter 3:
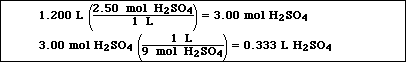
![]()
Measure 333 mL of 9.00 M H2SO4 and add the solution to
approximately 600 mL of distilled water. Remember always add acid to
water when diluting a concentrated acid or base solution. Then add
enough water to the diluted solution to obtain a final volume of 1200
mL of solution.
APCBS Exam II PAGE 5
![]()
(10) Solve ONE of the two problems in this part. (A second problem will not be scored.)
![]()
7. A 50.00 g sample of 0.600 M NaOH(aq) at 22.00 ºC is added to a coffee-cup calorimeter containing
50.00 g of 0.600 M HCl(aq) at 22.00 ºC. If the heat capacity of the calorimeter is 50.0 J/ºC and the
specific heat of the solution is the same as that of water (4.184 J/ºC) and the final temperature of the
solution in the calorimeter is 25.40 ºC, calculate the heat released in the reaction.
Very similar to Exercise 9c in Chapter 4:

![]()
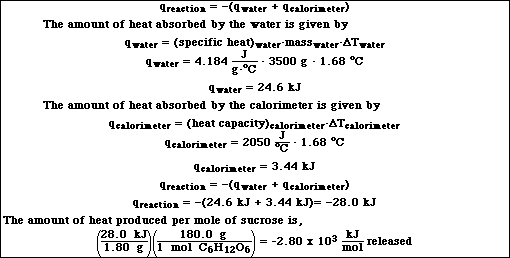
8. Glucose, C6H12O6 reacts with oxygen according to the reaction,
![]()
![]()
![]()
Calculate the heat produced per mole of glucose when 1.80 g of C6H12O6 are combused with excess
oxygen in a bomb calorimeter containing 3.50 kg of water. The temperature change measured is 1.68
ºC. The heat capacity of the calorimeter is 2050 J/ºC .
![]()
Identical to Exercise 11b in Chapter 4:
APCBS Exam II PAGE 6
![]()
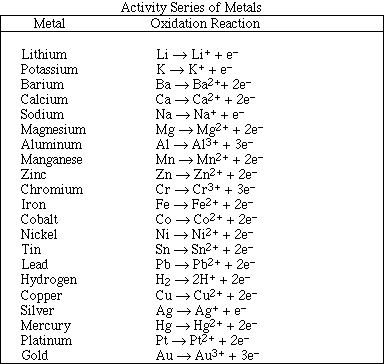
(12) 9. For each of the following reactions use the activity series to predict the products. If no reaction occurs
write NR. Balance the equations and identify the product phases as either (g)as, (l)iquid, (s)olid or
(aq)ueous. (Note: Points will be removed if the phases of the products are not provided.)
Very similar to Exercise 9a in Chapter 5:
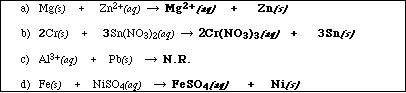
![]()
(5) 10. Compare and contrast the techniques of filtering and decanting. What factors must be considered in
determining which of these two techniques is the most appropriate for separating a given mixture?
Both filtering and decanting are used to separate heterogeneous mixtures. If the mixture contains a dense solid in a liquid, decanting may be adequate. If the mixture contains a suspended solid, filtering must be used to separate the mixture.
APCBS Exam II PAGE 7
![]()
Multiple Choice:
Print the letter (A, B, C, D, E) which corresponds to the answer selected.
11. B![]() 12. D
12. D![]() 13. E
13. E ![]() 14. B
14. B
15. A ![]() 16. A
16. A ![]() 17. A
17. A
ONLY THE ANSWERS IN THE AREA ABOVE WILL BE GRADED. Select the most correct answer for each question. Each question is worth 3 points.
11. Which of the following reactions is not exothermic?
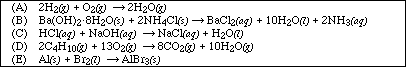
12. The addition of 3.31 kJ of heat to a 300. g sample of mercury at 19.0 ºC caused the temperature to rise to 99.0 ºC. What is the specific heat of mercury?

13. Which of the following equations is an example of a standard formation reaction?
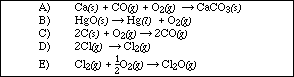
14. For which of the following reactions is DE = DH?
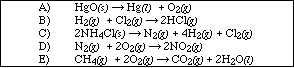
APCBS Exam II PAGE 8
![]()
15. Given the reaction,
![]()
![]()
If 25.2 g of FeSO4, 11.6 g of K2CrO4, and 23.5 g H2SO4 are mixed together, which reactant is the
limiting reagent? (Molar masses:
FeSO4 = 152 g;
K2CrO4 = 194 g;
H2SO4 = 98.0 g)
![]()
A) FeSO4
B) K2CrO4
C) H2SO4
D) K2CrO4 and H2SO4
E) not enough information
16. Which of the separation techniques listed below would be the most efficient in separating a mixture of dyes in a single drop of sample?
A) paper chromatography
B) decantation
C) evaporation
D) filtering
E) column chromatography
![]()
17. A pure sample of an oxide of mercury with a mass of 0.856 g was completely decomposed to its
elements by heating, producing 1.976 x 10-3 moles of O2. What is the likely formula of this oxide?
![]()
A) HgO
B) Hg2O
C) HgO2
D) Hg2O3
E) Hg3O2