Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam I
September 18, 1990
![]()
INSTRUCTIONS:
1. This examination consists of a total of 8 different pages. The last page includes a periodic table and some useful information. All work should be done in this booklet.
2. PRINT your name, high school and teaching partner's name now in the space at the top of this sheet. DO NOT SEPARATE THESE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. You do not have to show your work for the multiple choice or short answer questions.
4. No credit will be awarded if your work is not shown in problems 4, 5 and 11.
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()
APCBS Exam I PAGE 2
![]()
(8) 1. Write the chemical formula(s) of the product(s) and balance the following reactions which were
demonstrated in lecture. Identify all products phases as either (g)as, (l)iquid, (s)olid or (aq)ueous.
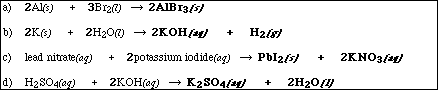
![]()
(6) 2. List at least two physical properties for each of the following substances.
![]()
a) Cl2
Greenish-yellow
Gas
![]()
b) S8
Yellow
Solid
![]()
c) sodium
Lusterous silvery
Solid
![]()
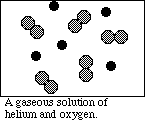
(4) 3. Diagram each of the following systems as viewed at the atomic level in the space provided.
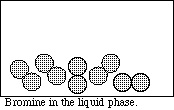
APCBS Exam I PAGE 3
![]()
(12) 4. Complete the following conversions. Note: Report the answer to the correct number of significant
figures.
a) 169 pounds to kilograms
![]()
![]()
![]()
b) 72.5 ºF to ºC

![]()
c) 27.0 ft3 to m3
![]()
![]()
![]()
(6) 5. Isopropyl alcohol has a density of 0.786g/cm3. Calculate the mass of isopropyl alcohol in a 1.00 gallon
bottle.
![]()
![]()
![]()
(4) 6. Complete each calculation and report the answer to the correct number of significant figures.

APCBS Exam I PAGE 4
![]()
(8) 7. Complete the following table;
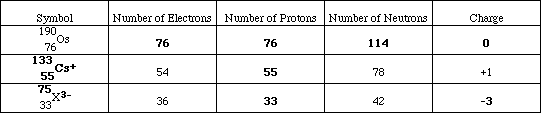
What is the symbol and the correctly spelled name for the unknown element, X?
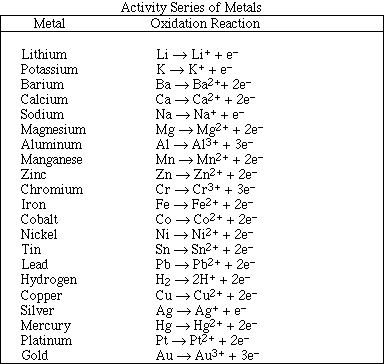
As : Arsenic
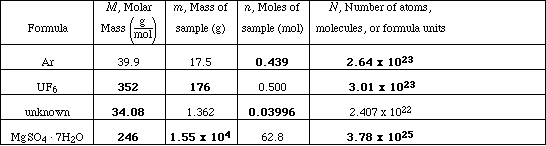
(10) 8. Complete the following table
![]()
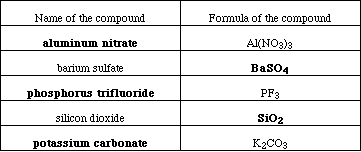
(5) 9. Complete the following table;
APCBS Exam I PAGE 5
![]()
(4) 10. The following chemical equation appeared as written below in an issue of a chemical journal reporting
current research findings.
![]()
a) Explain why this is not a balanced equation.
There are more potassium atoms in the products than reactants.
![]()
b) In a later issue the authors apologized for a typographical error in one of the products. Which of
the products do you feel is incorrect? Write the correct formula.
![]()
K3HN(SO3)2 is most likely incorrect. A possible formula which would yield a
balanced equation is K2HN(SO3) 2.
![]()
(15)11. The chemical equation for the reaction between aluminum and hydrochloric acid is,
![]()
a) Balance the equation.
![]()
b) If 5.49 g of aluminum are combined with excess hydrochloric acid calculate the mass of hydrogen
produced.
![]()
![]()
![]()
c) If the hydrogen produced in the above reaction was reacted with excess oxygen how many grams
of water could be produced?
Reaction
![]()
![]()
![]()
APCBS Exam I PAGE 6
![]()
Multiple Choice:
Print the letter (A, B, C, D, E) which corresponds to the answer selected.
12. A ![]() 13. A
13. A![]() 14. B
14. B![]() 15. C
15. C
16. C![]() 17. E
17. E
ONLY THE ANSWERS IN THE AREA ABOVE WILL BE GRADED. Select the most correct answer for each question. Each question is worth 3 points.
12. Which of the following conclusions can be drawn from J. J. Thomson's cathode ray experiment?
A) Atoms contain electrons.
B) Practically all the mass of an atom is contained in its nucleus.
C) Atoms contain protons, neutrons and electrons.
D) Atoms have a positively charged nucleus surrounded by an electron cloud.
E) The charge on an an electron was equal and opposite to the charge on a proton.
13. Magnesium reacts with a certain element to form a compound with the general formula MgX. What would the most likely formula be for the compound formed between potassium and element X?
![]()
A) K2X
B) KX2
C) KX3
D) K2X3
E) K3X2
![]()
14. The relative atomic mass of one of two naturally occurring isotopes of boron is 10.013 u. The
percentage abundance of this isotope, 10B, is 19.61 %. What is the relative atomic mass of the other
naturally occurring isotope of boron?
A) 10.8 u
B) 11.0 u
C) 11.9 u
D) 12.0 u
E) There is not enough information to answer the question.
15. Djenkolic acid, which can be isolated from beans, is possibly linked to flatulence. It is 33.06% carbon, 5.55% hydrogen, 11.01% nitrogen, 25.16% oxygen and 25.22% sulfur. Determine the empirical formula for djenkolic acid.
![]()
A) C6HN2O5S5
B) C3H7NO2S
C) C7H14N2O4S2
D) C7H7NO2S2
E) C3H5NO2S2
APCBS Exam I PAGE 7
![]()
16. How many oxygen atoms are contained in a sample of H2SO4 with a mass of 3.257 x 10-22 g?
![]()
A) 2
B) 4
C) 8
D) 196
E) 785
![]()
17. How many grams of calcium nitrate, Ca(NO3)2, contains 24 grams of oxygen atoms?
![]()
A) 164
B) 96
C) 62
D) 50.
E) 41
![]()