Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam V
December 18, 1990
![]()
INSTRUCTIONS:
1. This examination consists of a total of 9 different pages. The last 3 pages include a periodic table, useful mathematical equations and constants, a table of standard heats of formation, and an activity series. All work should be done in this booklet.
2. PRINT your name, high school and teaching partner's name now in the space at the top of this sheet. DO NOT SEPARATE THESE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. You do not have to show your work for the multiple choice or short answer questions.
4. No credit will be awarded if your work is not shown in problems 3, 6, 7, and 8.
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()

APCBS Exam V PAGE 2
![]()
(12) 1. Write the chemical formula(s) of the product(s) and balance the following reactions which were
demonstrated in lecture. Identify all products phases as either (g)as, (l)iquid, (s)olid or (aq)ueous.

![]()
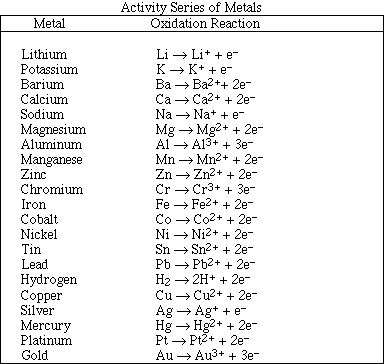
(6) 2. For each of the following reactions use the activity series to predict the products. If no reaction occurs
write NR. Balance the equations and identify the product phases as either (g)as, (l)iquid, (s)olid or
(aq)ueous. (Note: Points will be removed if the phases of the products are not provided.)
![]()
![]()
(6) 3. Complete the following conversions. Note: Report the answer to the correct number of significant
figures.
a) 0.346 mg to kg
![]()
![]()

![]()
(3) 4. Complete the calculation and report the answer to the correct number of significant figures.
![]()
APCBS Exam V PAGE 3
![]()
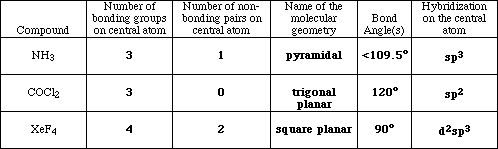
(15) 5. Complete the following table
![]()
(10) 6. Calculate the density of SF6(g) at STP.
![]()

APCBS Exam V PAGE 4
![]()
(12) 7. The DHºrxn for the reaction
![]()
![]()
is +164 kJ.
![]()
a) Calculate DHºf for HCN(g).
![]()

b) Write the standard formation reaction for HCN(g).
![]()
![]()
(10) 8. The normal boiling point for n-octane is 126 ºC. At 59.0 ºC the vapor pressure of n-octane is 100.0 mm
Hg. Calculate DHvap for n-octane.
![]()
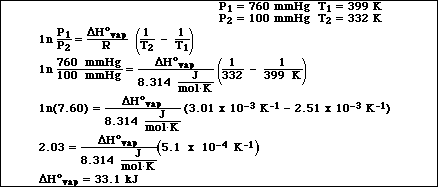
APCBS Exam V PAGE 5
![]()
(6) 9. The van der Waals equation for real gases is,
![]()
Two constants a and b are added to account for the behavior of real gases. Briefly describe the physical significance of each correction.
![]()
a is a constant unique to each gas and has units of L2.atm/mol2 which corrects for the
forces of attraction which occur between gas particles. The term an2/V2 is the
correction factor to the pressure to correct for forces of attraction.
nb is a correction factor for the finite volume of the gas particles. Real gases have a volume less than the total volume to move in. So the actual volume is slightly less and is determined by the constant b. The value of b is unique to each gas.
![]()
(10)10. In the space below sketch the phase diagram for water. Identify each of the three phase areas, the three
phase change lines and two important points. Briefly explain a novel feature in the phase diagram for
water.
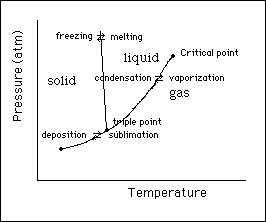
The slope of the freezing ä melting line has a negative slope which is unique to water. The negative slope occurs because the solid phase of water is less dense compared to the liquid phase.
APCBS Exam V PAGE 6
![]()
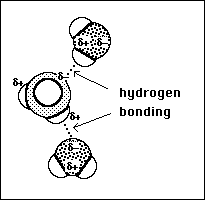
(10)11. The primary species present when ammonia dissolves in water is the molecule, NH3. Illustrate the
possible intermolecular interactions, by drawing a picture depicting the interaction at the microscopic
level, that can occur between NH3 and H2O. In your illustration clearly label and identify the primary
intermolecular force of attraction.