GROUP AND DISCUSSION ACTIVITIES
 Key Questions Key Questions
|
 Counterintuitive
Examples and Discrepant Events Counterintuitive
Examples and Discrepant Events |
 Pictures in the
Mind Pictures in the
Mind |
 Language of
Chemistry Language of
Chemistry |
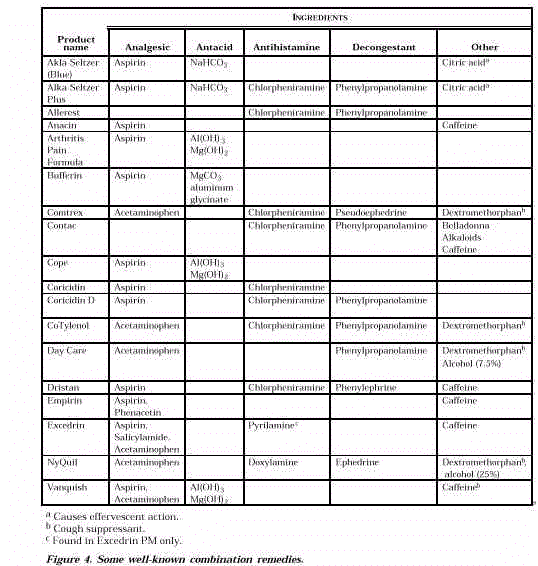
 Drug Listing Drug Listing |
 Pattern
Recognition Pattern
Recognition |
 Common Student
Misconceptions Common Student
Misconceptions |
 Classroom
Discussion Classroom
Discussion |
 History: On The
Human Side History: On The
Human Side |
 Humor: On The Fun
Side Humor: On The Fun
Side |
 Media Media |
Key Questions
Is water a drug? [When given therapeutically to a
dehydrated person, water can be considered a drug.]
Under what condition might aspirin not be considered a drug? [When taken by an anxious
insomniac, aspirin would have no drug effect.]
Using the four names for a common drug given here, identify the generic name, the street
name, the chemical name, and the brand name: blue birds, sodium pentobarbital, sodium
5-ethyl-5-(1-methylbutyl) barbiturate, Nembutal. [Generic name: sodium pentobarbital;
street name: blue birds; chemical name: sodium 5-ethyl-5-(1-methylbutyl) barbiturate;
brand name: Nembutal.]
A patient purchases a cough syrup consisting of 200 mL liquid. The label directs the
patient to take 1 teaspoonful four times a day. How long will the prescription last if 1
teaspoon is equivalent to 5 mL? [10 days]
If the usual dose of a sulfa drug for a urinary tract infection is 2.00 g/kg body
weight, how many grams of the drug should be given to a female if she weighs 120 lb (2.20
lb = 1 kg)? [109 g]
Examine the labels of three combination pain relievers (Bufferin, Excedrin, Vanquish,
etc.). Make a list of the ingredients of each. Look up the medical uses, dosages, side
effects, and toxicities of each in The Merck Index .
Counterintuitive Examples and Discrepant Events
- "My tooth stopped hurting when I got to the dentist’s office" or
"Doctor, I feel better just because you are here." The anticipation of
professional help is often reassuring to patients and provides support. It has much the
same effect as a placebo. It is believed that certain natural body (endogenous) drugs
(endorphins, adrenal hormones, and neurotransmitters) are released in this clinical and
verbal approach.
- Methanol (methyl alcohol) poisoning is treated by intravenous injection of ethanol
(ethyl alcohol). Ethanol is oxidized more easily by the enzyme that catalyzes the
oxidation of methanol in the body, and methanol is excreted unchanged in the urine. Enzyme
oxidation of methanol yields formaldehyde and formic acid, both highly toxic substances.
By saturating the system with ethanol, methanol cannot compete successfully for the enzyme
and will be excreted largely unchanged.
- More is better (a vitamin ad). Ingestion of large dosages of vitamins have been touted
as being highly beneficial. Linus Pauling, twice winner of the Nobel Prize, advocates
large daily doses of Vitamin C. While the beneficial effects of this practice remain in
doubt, at least it should do no harm since Vitamin C is water soluble and will be quickly
excreted. The fat-soluble vitamins, however, are not so quickly excreted and can end up in
the body’s fat reserves. Thus, overdosages of fat-soluble vitamins (e.g., Vitamin A)
have been documented in the medical literature and can lead to serious problems, even
death.
Pictures in the Mind
- TV Public Service message: "This is your brain (an egg); this is your brain on
drugs (egg on a hot grill)."
- TV Public Service message: "Before you get on drugs, you’d better know what
you’re jumping into (diver plunges into an empty swimming pool)."
T
IPS FOR THE TEACHER
Language of Chemistry
1. Classification of drugs.

- Addiction, dependence, and tolerance are three terms used in describing the effects of
some substances. Addiction is the continued need of a substance to
function normally and to prevent the occurrence of withdrawal symptoms. Dependence
is experience of withdrawal symptoms if use of the substance stops. Tolerance
is the need for increasing amounts of a substance to experience the desired effect.
- Chemotherapy is a planned attack on a disease using a specific
chemical. Such substances have been designed to attack the disease center (not just
relieve the symptoms), do their job and not harm healthy tissue. Substances have been
prepared for that very purpose (see also Drug Design in Links and Connections section).
Some chemotherapeutic agents and the disease they attack are: Salvarsan, syphilis;
antibiotics, bacterial infections; radioactive iodine, thyroid cancer; and
antimetabolites, some cancers. The possibility of side effects from the use of
chemotherapy is a major problem.
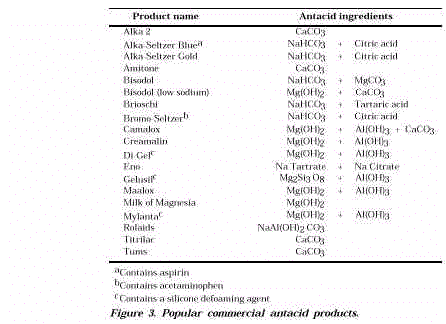
- An antacid is an alkaline substance that reacts with excess stomach
acid (HCl). As shown in Figure 3, there are many varieties of over-the-counter (OTC)
antacids. The most common antacid ingredients are sodium bicarbonate (NaHCO 3 ), calcium carbonate (CaCO 3 ),
magnesium carbonate (MgCO 3 ), aluminum hydroxide
(Al(OH)3 ), and magnesium hydroxide (Mg(OH)2 ). The carbonate antacids neutralize excess hydrochloric
acid with the liberation of CO 2 .
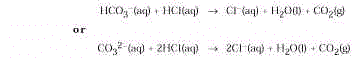
- Analgesics (pain relievers) in common use
today are aspirin, acetaminophen, and ibuprofen. All are available as over-the-counter
(OTC) preparations. Of the three, aspirin is the only analgesic (reduces pain),
antipyretic (reduces fever), and anti-inflammatory (reduces swelling). Acetaminophen is
both an analgesic and antipyretic. Acetaminophen has been used as a combination pain
reliever with codeine. Ibuprofen is an analgesic. The structure of each is shown below:
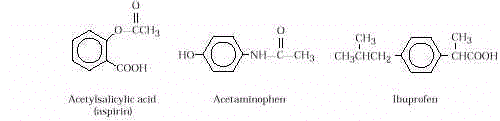
Typical doses of
aspirin and acetaminophen are 325mg and for ibuprofen, 200mg. These drugs have drawbacks.
Aspirin causes intestinal bleeding and may cause
allergies.
It has been implicated in Reye’s syndrome, a life-threatening condition that may
follow flu or chicken pox in children and teenagers. Acetaminophen is
not useful
to patients with arthritis, and it is toxic to the liver in large doses. Ibuprofen causes
intestinal bleeding and impairs kidney function.
- Narcotic analgesics are used to obtain higher levels of pain relief
(e.g., the pain associated with fractures, kidney stones, heart attack, gallstones) than
can be achieved with the OTC aids. They produce stupor or insensibility as well as
addiction and withdrawal symptoms and increasing tolerance. Narcotic analgesics include
codeine, morphine, and meperidine (Demerolď).
Morphine is found naturally in the opium poppy. Codeine is made by methylating
(substituting the CH 3 – group for H–)
morphine. Meperidine is a synthetic narcotic. The dose for pain relief of these analgesics
is typically 5–120mg.

- Combination pain relievers contain aspirin and caffeine. Caffeine is a
mild stimulant found in coffee, tea, and cola drinks. Evidence indicates that caffeine
counteracts the action of aspirin. Thus combination pain relievers containing caffeine are
less effective. Some combination pain relievers contain an antacid.
- Colds are caused by viruses, and antibiotics are not effective against cold viruses. Cold
remedies treat only the symptoms and may contain a cough suppressant, a nasal
decongestant, and an antihistamine. The cough suppressant is either codeine or
dextromethorphan. The antihistamine, usually diphenhydramine or chlorpheniramine, relieves
allergy symptoms (sneezing, itchy eyes, and runny nose). The nasal decongestants are
usually ephedrine and phenylephrine. These drugs have been found to be safe and effective
when taken as directed, but antihistamines can cause drowsiness and are potentially
hazardous to anyone who needs to be alert. Abuse of cold remedies can lead to addiction.
- Some well-known combination remedies are shown in Figure 4.
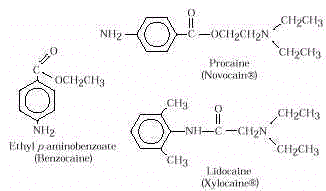
- Local anesthetics render one part of the body insensitive to pain.
Benzocaine is available in some OTC preparations for localized surface pain and itching.
Procaine and lidocaine are more potent and are used in dental repair or for minor surgery.
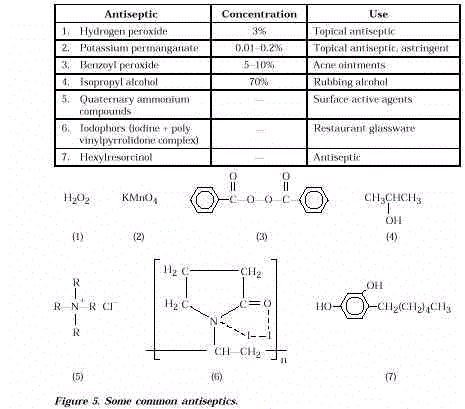
- Antiseptics are compounds that are applied
to living tissue to kill microorganisms or to prevent their growth. Many are mild
oxidizing agents and are effective because they oxidize bacterial cells (as well as human
cells) or weaken cell walls so that the cell contents cannot be contained (see Figure 5).
- Vitamins are organic compounds required in small amounts in
the diet because they cannot be synthesized by the body. Lack of proper amounts of
vitamins in the diet can lead to vitamin-deficiency diseases. For example, Vitamin A
deficiency leads to nightblindness (poor vision in dim light), Vitamin D deficiency lead
to rickets (soft and poorly mineralized bones), and Vitamin K deficiency leads to
hemorrhaging. Some vitamins are necessary in making certain coenzymes. For example,
riboflavin is needed for flavin adenine dinucleotide (FAD), and niacin is needed for
nicotinamide adenine dinucleotide (NAD). These particular coenzymes are involved in
oxidation-reduction reactions. Mega doses of vitamins, unless prescribed by a physician,
are dangerous.
- Antibiotics are derived from molds or bacteria and inhibit
the growth of other microorganisms. Discovered in 1941, penicillin is made by the mold
Penicillium notatum. Many different penicillins exist, differing only in the structure of
the R group in the general formula shown below. The nature of the R group determines
whether or not the penicillin can be taken orally or by injection, and whether it causes
diarrhea or an allergic reaction.

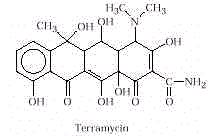
Tetracycline antibiotics get their name from
their four-ring structure. First used in 1950, they are effective against a wide variety
of microorganisms. Initially obtained
from fungi, many tetracyclines are
synthesized today. Terramycin is a typical example.
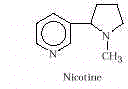
- Nicotine and Smoking. People smoke for enjoyment, to alleviate stress,
or because they are addicted to nicotine. Yet nicotine, the active ingredient in tobacco,
is toxic. It is lethal at doses of about 60mg, and a typical cigar contains at least
120mg. However, only about 10% of that amount is absorbed on inhaling the smoke, and over
a relatively long period. A typical filter cigarette contains 20-30mg of nicotine. Low
doses of nicotine increase respiratory rate, stimulate an increased heart rate and blood
flow, and constrict the arteries. Regular smokers exhibit a shortness of breath because CO
and HCN formed in the combustion processes bind to hemoglobin, diminishing its ability to
carry oxygen. At high doses, nicotine interferes with nerve impulse transmission.
Tolerance can be developed for small amounts of nicotine along with a dependency.
It is not just the nicotine in cigarettes that is harmful.Benzpyrene, found in tobacco
smoke, is an extremely carcinogenic compound. Tobacco also contains b-naphthylamine, which causes bladder cancer.
Individuals chewing or dipping smokeless tobacco have a particularly dangerous habit.
Smokeless tobacco causes tooth decay (it has a high sugar content), gum disease, and oral
cancer. Nicotine levels in the blood of smokeless tobacco users is comparable to or
greater than cigarette smokers.
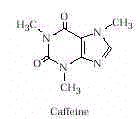
- Caffeine is found in tea, coffee, and cola drinks. Caffeine is an
alkaloid (i.e., can be extracted from its natural source by acid) and a
stimulant. It causes an increased alertness, the ability to put off sleep, and an
increased capacity for thinking, but coordination and timing may be adversely affected. It
is the main ingredient in certain OTC products, such as No-Doz (100mg caffeine per dose)
and Vivarin (200mg per dose). Caffeine users can develop tolerance and dependency. Coffee
drinkers (90mg/ cup) will experience lethargy, headache, or nausea after abstaining for up
to 15 hr. Cola drinkers (43mg/can) and tea drinkers (50mg/ cup) can also experience these
reactions.
- Ethanol. Alcohol is a term that describes a
class of organic compounds with a hydroxy group (–OH) bonded to a carbon atom. It is
also a term that the populace uses to identify liquors, beers and wines. The alcohol
contained in these and other alcoholic beverages is ethanol (CH 3 CH 2 OH). Beer is usually 6% ethanol
by volume; wines, 12%; and 80 proof whisky, 40%. (Proof = 2 x alcohol content by volume.)
Ethanol is a mild depressant slowing down physical and mental activity in moderate
amounts. In very large amounts, it produces unconsciousness or death. Although
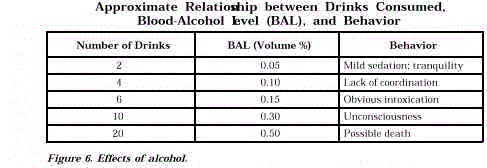
alcohol’s effects vary among individuals, usual effects are summarized in Figure 6.
The relationship applies to a 70 kg (154-lb male) who rapidly consumes 30-mL (1-oz) shots
of 90-proof (45% ethanol) whiskey.
Ethyl alcohol is detoxified by a liver
enzyme through an oxidation reaction. In individuals who develop tolerance for alcohol,
increasing amounts of the enzyme build up
in the liver enabling large amounts
of alcohol to be detoxified.
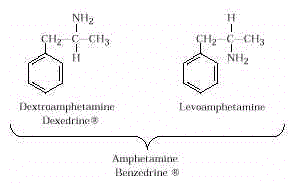
- Benzedrine is a mixture of two optical isomers of amphetamine. These
two isomers differ in the way they rotate plane polarized light. These isomers occur
because of the four different groups (C 6 H 5 CH 2 –, NH 2 –, CH 3 –, and
H–) around the central carbon atom. Dexedrine is the stronger stimulant of the two.
Amphetamines are central nervous system stimulants that increase heart rate and blood
pressure. They increase wakefulness, drive, and energy. They produce a temporary elevation
of mood, usually followed by fatigue, irritability, and depression. Users often must take
barbiturates to sleep. Amphetamines have been used for weight reduction and to relieve
mild depression. In colloquial language, amphetamines are called "uppers."
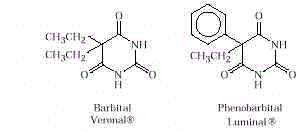
- Barbiturates, colloquially called
"downers," are used to produce mild sedation (a few milligrams) and to induce
sleep (about 100mg). They are the major ingredient in sleeping pills and can cause death
in the case of an overdose. Barbiturates are addictive. Users generally develop a
tolerance and require larger doses to obtain the desired degree of sedation. Structurally,
barbiturates are amides. Barbiturates are particularly dangerous when used along with
alcohol. Both substances are depressants, but when used together the sum of the effects is
magnified (synergism). The structures of common barbiturates are given below.
- Tranquilizers are used to calm nerves and
relieve tension. Cope, Vanquish, and Compoz are OTC drugs available to help individuals
"cope" with problems and unwind. These products also contain aspirin and an
antihistamine, which typically causes drowsiness. Tranquilizers are also available by
prescription (for example, Equanil, Valium, and Librium). Many tranquilizers are addictive
and attempts to stop using them have causedwithdrawalsymptoms.
Morepotenttranquilizers(e.g., thorazine)are useful in treating severe forms of mental
illness and schizophrenia. The structure of some common tranquilizers are given.

- Radiopharmaceuticals . Radioisotopes are used for therapy and diagnosis
in medicine. As a diagnostic tool, they provide information about the type or extent of
illness. The therapeutic use is intended to treat or cure a disease. Some examples are
shown in Figure 7.

Both diagnostic and
therapeutic radioisotopes typically produce gamma radiation. In low doses, gamma radiation
is less harmful to tissues than alpha or beta
radiation.
Although alpha and beta radiation produce more cell deaths per unit of exposure than gamma
radiation, the penetrating power and ranges of alpha and
beta
radiation are very small. Gamma radiation penetrates tissues very effectively and, in high
dosages, is very effective in causing cell death, particularly in rapidly
multiplying
cells. For this reason, gamma radiation is used for therapeutic purposes as well.
Typically a beta emitter like cobalt-60 is used for cancer treatment. An
atom of
cobalt-60 transmutes to a highly excited atom of nickel-60, which emits two high-energy
gamma rays in quick succession. These gamma rays have been
found to be
effective in the treatment of some types of cancer.
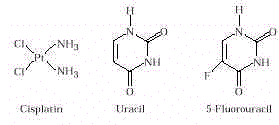
- Anticancer Drugs. Cancer is cell multiplication without restraint with
invasion of nearby tissue. In addition to surgical removal of affected areas and
irradiation to kill the cancer cells, cancers are treated by chemotherapy use of chemical
substances to kill cancer cells. These substances include alkylating agents, cisplatin,
and antimetabolites. Alkylating agents are organic compounds that transfer alkylating
groups to nitrogen bases (usually guanine) in DNA. The presence of the alkyl group in
guanine prevents DNA replication (and thus, cell division). Cisplatin also blocks DNA
replication. Antimetabolites interfere with synthesis of a nucleotide necessary for DNA
synthesis and therefore inhibit cell division. 5-Fluorouracil is an antimetabolite (see
Drug Design in Links and Connections section). The use of these substances is based on the
premise that cancer cells are undergoing rapid division and will be affected to a greater
extent than healthy cells. In the long run, healthy cells suffer as well. Chemotherapy has
provided an improvement in patient survival with some cancers, particularly breast,
bladder, and colon cancer, and Hodgkin’s disease.
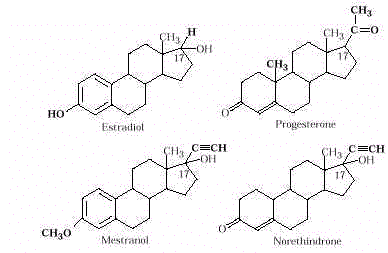
- Birth control pills are steroids inhibiting
ovulation and "convincing" the body that it is pregnant. If an egg is not
released, females cannot become pregnant. The pill, a synthetic hormone-like substance,
was first used in 1960. Formostfemalesthe pill is safe, but some women experience
undesirable side effects: hypertension, acne, and abnormal bleeding. A combination pill
that contains both synthetic estrogen and progesterone is typically used. Notice the
structural similarity of these synthetic compounds (mestranol and norethindrone) to the
female sex hormones progesterone and estradiol. Substitution of the –C║CH group for a –CH 3 group
at C-17 allows for a more effective drug (see Drug Design in Links and Connections
section).
- Anabolic steroids are synthetic derivatives
of testosterone. These drugs are used in therapy to correct hormonal imbalance or to
prevent withering of muscle in persons who are recovering from surgery or starvation.
Healthy athletes use these drugs in doses 10 times the therapeutic dose to build muscle
mass. When so used, a number of side effects are noticed including acne, baldness,
enlargement of the breasts, and changes in sexual desire. In women, use of anabolic
steroids produces facial hair, deepening of the voice, and changes in the menstrual cycle.
Anabolic steroids are toxic to the liver. Notice the structural relationship between
testosterone and fluoxymesterone, a typical anabolic steroid. The presence of the –CH
3 group at C-17 is thought to slow the metabolism of
the steroid in the liver and increases liver toxicity.

- The following reference guide to some drugs that are subject
to abuse is available from Council on Drug Abuse, 698 Weston Road, Toronto, Ontario, M6N
3R3; (416) 763-1491.
* See  Drug Listing page for
the above mentioned informaiton *
Drug Listing page for
the above mentioned informaiton *
Pattern Recognition
Drugs are not good or bad in themselves. They may be used properly or abused.
Similarities in structural formulas of drugs cause similar physiological responses.
Common Student Misconceptions
- "Bufferin is twice as fast as aspirin. Bufferin helps prevent the stomach upset
often caused by aspirin." The FDA investigated these advertising claims and did not
find the claims to be true.
- "Chicken soup is the best cure for the common cold." There is no scientific
evidence that chicken soup can cure the common cold. Plenty of liquids and rest, and 5-7
days of suffering are the best cures.
- "One of my friends took a drug once and got better." This statement is
nonspecific and says nothing about drug effectiveness.
- "There is only one kind of alcohol." The "one kind of alcohol"
typically thought of is ethanol or ethyl alcohol. Methyl alcohol and isopropyl alcohol are
other alcohols.
- "More is better." This typically refers to minerals and vitamins that are
needed by the body in small amounts. Taking megadoses of these substances can cause some
unexpected problems. For example, too much vitamin D can cause pain in bones, nausea and
diarrhea, and weight loss. Too much salt (NaCl) in the diet can cause elevated blood
pressure in some individuals.
- "Walking across a field of poppies will put you to sleep." (From the Wizard of
Oz.) When Dorothy and her friends in the Land of Oz walked across the poppy fields, they
fell asleep. Although opium poppies contain morphine, a central nervous system depressant,
walking through a poppy field will not cause one to fall asleep. The morphine must be
extracted; in situ it is nonvolatile.
- "Antiseptic, antibiotic, analgesic, and anti-inflammatory mean the same
thing." These terms do not mean the same thing and the distinction among them was
described in this module.
- "Two extra strength aspirin are better than 3 regular aspirin." Three regular
aspirin contain 975 mg (3 x 325 mg) of active ingredient. Two extra strength aspirin
contain 1000 mg (2 x 500 mg) of active ingredient. The difference (25 mg) is not enough to
make a significant difference.
- "Receiving a vaccine guarantees immunity." A vaccine is designed to cause a
person to build a resistance to a viral infection (e.g., mumps, polio). When given a
vaccine, some individuals have developed serious cases of the disease that the vaccine is
designed to alleviate.
Classroom Discussion
Classroom discussion is particularly important in this module. Suggested points for
discussion are indicated below.
- Why do people smoke or use drugs if we know that they are bad for you? (The discussion
will avoid judgmental aspects and will address the social problem.)
- How is the legal use of alcohol and cigarettes different from the illegal use of
pleasure drugs?
- To what extent is the use of anesthetics warranted in ordinary cuts and bruises,
dentistry, and childbirth?
- At what point in an illness should the decision to use or terminate chemotherapy occur?
H
ISTORY: ON THE HUMAN
SIDE
For centuries, humans have eaten or chewed berries, bark, roots, and herbs to alleviate
or cure sickness or disease. The origin of disease was unknown, and its treatment was
coupled with magic and superstition. However, the scientific practice of medicine began in
ancient Asiatic civilizations. The first known code of medical ethics was established in
Sumer. The ancient practice of acupuncture and ideas about blood circulation suggest that
the Chinese were familiar with anatomy, vascular systems, and the nervous system. In
addition to introducing the Hippocratic oath, the Greeks advanced medical knowledge in
anatomy and physiology, diet, and exercise. Through their sanitation facilities, the
Romans improved public health. Medical knowledge began to decline during the Middle Ages
in Europe, but Arab and Jewish physicians made some progress. For example, two Persians
introduced opium for coughs and extracts of crocus seeds to treat gout. Both of these
remedies are in use today.
The ancients knew that chewing willow tree (Genus: Salix) bark had an analgesic effect.
Centuries later, the active ingredient was isolated from the willow bark and called
salicylic acid in deference to its origin.
Jesuit missionaries introduced an important herbal remedy into Europe in the 17th
century. This remedy was an extract of cinchona bark obtained from South American Indians.
The extract was used by Indians against chills and fever and soon became a favorite
anti-malarial medicine in Europe. The principal ingredient of cinchona bark is quinine.
Also, in the 17th century, William Harvey demonstrated blood circulation and the heart as
a pump. A major step toward diagnosing disease was made with the introduction of the
compound microscope, making minute forms of life visible for the first time. The 18th
century witnessed the introduction of vaccination by Edward Jenner. Modern medicine had
its birth in the 19th century with the development of the germ theory of disease, the use
of anesthesia (Crawford Long, 1842), antiseptics in surgery (Joseph Lister, 1867), and a
revival of public health measures and better sanitation. During the 20th century there has
been an increased understanding of immunity, the endocrine system, and the importance of
vitamins and nutrition, advances in surgery, organ transplants, and diagnostic techniques
(CAT scan, MRI, etc. ). Chemotherapy is an important development of the 20th century.
Specific drugs have been discovered and designed to eliminate disease-causing
microorganisms (antibiotics), to rectify a hormonal imbalance (insulin), and to arrest a
cancerous growth (anticancer drugs).
The following are three interesting stories related to chemistry in medicine. The
first two are taken from Men of Medicine by Katherine Shippen (1957). The last is from
Medical Heroes and Heretics by Wayne Martin (1977).
Who Discovered Anesthesia?
In the eighteenth century, the English chemist Humphry Davy made dinitrogen oxide (N 2 O, nitrous oxide, laughing gas), and observed that it
prevented feeling physical pain. He thought it might be useful in surgery, but did nothing
about it. It wasn’t until the next century that a true anesthetic was made, although
its discoverer has not yet been satisfactorily determined.
As towns sprang up in the West and South of the United
States, people were entertained by showmen who featured laughing gas in their repertoire.
At one of these laughing gas shows in December 1841, the Georgia physician Crawford Long
observed its effects first-hand. Long was very popular with young people, and when his
young friends asked him to prepare them some laughing gas, he told them he lacked the
apparatus for making it but that he had a medicine called sulfuric ether that would do as
well. He sometimes gave a few drops of this compound to nervous patients. Some of his
friends tried inhaling sulfuric ether, others soon followed suit, and before long ether
parties were being held all through the county. People affected by the gas would throw
themselves about hilariously, knocking against furniture, often falling down, bruising
themselves, and scraping shins and elbows. Although the cuts and bruises were often
considerable, the participants at the ether frolics never complained of them. They
didn’t even seem to notice that they had hurt themselves. Long correctly reasoned
that the ether prevented their feeling pain.
Long decided to use the ether in an operation to remove two
small tumors on the back of the neck of a young patient. He let the patient inhale some
ether and, after the muscles completely relaxed, removed the tumor. Long recorded the
surgery in his ledger, but did not report this first surgery under ether (1842) to a
medical journal. Whatever the reason, it was a shock to him to read in a medical journal
four years later that William Morton in Boston had administered a gas to a patient that
made him insensible to pain.
Morton received much acclaim for his discovery and Long
grew bitter over his neglect in reporting his own discovery. When he served in the Civil
War, Long kept his ledger with the entry of his discovery with him. Upon his return home
after the war, he placed the ledger in a trunk in the attic. His family never dared to
speak of the discovery since the mention of it distressed Long too much.
Meanwhile, Morton received permission to demonstrate his
ether in surgical operations before the great surgeons at Massachusetts General Hospital.
The doctors were interested in seeing the preparation that diminished the sensibility to
pain. Morton administered the drug to a patient who shuddered a little and then fell into
a deep sleep. Morton removed a large tumor of the jaw, and the patient did not stir as the
knife cut into his flesh. After regaining consciousness, the patient said the surgery
hadn’t hurt at all. Oliver Wendell Holmes, the poet and physician, suggested the word
"anesthesia" to Morton in late 1846. According to Holmes, the term signified
insensibility to touch.
Discovery of Penicillin
In 1914, Alexander Fleming, physician in the Royal Army Medical Corps, observed that
many soldiers wounded in combat developed blood poisoning, pneumonia, or some other
bacterial infection. Fleming knew there was need for an effective antiseptic to fight the
infections, but none was available.
After the war, Fleming began to teach bacteriology at St. Mary’s Hospital in
London. He spent many hours in the laboratory searching for the microbe-destroying agent
he had needed during the war. One day in 1928, he observed through his microscope that a
mold fleck had settled on one culture plate of bacterial growth sitting next to an open
window. At first he was annoyed that the mold had contaminated the plate. About to throw
it away, he stopped and examined the plate again. The mold was being surrounded by a halo
of watery fluid, and he surmised that something in the mold was destroying the microbes.
He removed the mold and grew more of it. At first it was fuzzy white, then it turned
green, spreading fronds that matted together in a thick mass. Fleming and his assistants
continued to study the watery fluid secreted by the mold and found that it was effective
against germs causing blood poisoning, sore throat, some types of pneumonia, but not
typhoid fever or dysentery. It did not affect blood serum as he had feared it might, nor
did it harm mice and rabbits. He concluded that it could be applied to the infected
surface undiluted, and it was nonirritating and nontoxic. He named the new antiseptic
penicillin, after the penicillium family to which the mold belonged.
In 1929, Fleming published a paper announcing the discovery of penicillin. The tiny
amounts of penicillin that Fleming could make in the laboratory were not enough to treat a
single patient, so his discovery did not arouse much interest. His paper lay forgotten on
the library shelves for nearly 10 years until, in 1939, an Australian physician, Howard
Florey, reread it and contacted Fleming. Fleming sent Florey some of the original mold,
and from it Florey eventually isolated about a teaspoonful of penicillin—enough to
treat one case of infection. He soon had the opportunity to find out if it worked: as a
last resort he administered the penicillin to a policeman with a severe case of blood
poisoning. The penicillin combated the blood poisoning effectively as long as it continued
to be administered, but the single teaspoonful was not enough. When the penicillin ran
out, the blood poisoning returned, and the patient died. Florey knew that larger amounts
of penicillin would have to be made on a commercial scale. Because England was involved in
a war in 1941, Florey went to the United States where penicillin could bemass-produced. By
1943, it was being used to treat infected wounds of the armed forces. Since that time,
many lives have been saved by this so-called "miracle drug." Fleming and Florey
shared the Nobel Prize for medicine in 1945 for their discovery.
Jonas Salk and the Polio Vaccine
In 1921, while vacationing on the Island of Campobello off the coast of Maine in New
Brunswick (Canada), Franklin D. Roosevelt was stricken by paralytic polio and was crippled
for life. In 1926, he established the nonprofit Warm Springs (Georgia) Foundation for
treating polio victims. The March of Dimes program, which was associated with the
foundation, amassed over $500 million to help polio victims, designating some $40 million
to finance polio research. In this research endeavor, the conflict of heresy and
established belief developed, yet both produced results. The heretic was Jonas Salk. His
heresy was the use of a killed-virus vaccine. In 1908, Karl Landsteiner (Austria)
established that polio was caused by virus. By 1941, there were three general types of
polio virus had been identified; it had also been determined that all strains of one type
would immunize against strains of that type only. By 1952, it was known that polio virus
circulated in the blood, and this fact proved to be its Achilles heel since a
vaccine-produced antibody in the blood could protect against polio. Isabel Mountain
prepared a killed vaccine using formaldehyde to inactivate the virus. She discovered that
the killed-virus vaccine would produce a degree of immunity in a monkey without harm.
Given these bits and pieces, what was Jonas Salk’s claim to fame? Salk put all the
pieces needed for eradication of the disease together by acting quickly and with great
precision. In 1947, Salk, then 33, was Research Professor of Bacteriology and Pathology at
the University of Pittsburgh. He undertook a program of typing the approximately one
hundred strains of polio virus that had been isolated. He became bored during the latter
part of the three-year program and began to look ahead to develop a vaccine based on the
knowledge available to him. The typing program confirmed that their were only three types
of polio virus. In 1951, Salk made a killed-virus vaccine—by treating polio viruses
with formaldehyde at 36 ░C for 11–13 days. This
killed-virus vaccine produced a high degree of immunity to all three types of polio in
monkeys contrary to the belief at the time that a killed-virus vaccine could not mobilize
antibodies. Salk continued to run tests to ensure that his vaccine was safe by repeatedly
determining that the vaccine contained no live virus. He tested his vaccine in immune
children (who had had a previous paralytic infection of polio). In all cases some increase
in antibody production was noted. In 1953, confident of his vaccine, Salk tested himself,
his family and about 400 volunteers in Pittsburgh. One year later the vaccinated subjects
were retested and found to have the same high level of vaccine-induced antibodies.
Children were vaccinated before the polio seasons of 1954 and 1955. The test showed the
vaccine to be safe and effective.
The medical establishment, led by Albert Sabin, complained about the use of the
killed-virus vaccine. Sabin claimed that if live virus remained in the vaccine and it were
used, it would kill and cripple. Sabin and his colleagues felt that use of a weakened
live-virus vaccine was preferable. Throughout his preparation of the killed-virus
vaccine,Salk made every effort to ensure that no live virus was present. Yet
unintentionally, Salk hurt many feelings and stepped on many tender toes in the process.
The ill will of the scientific community hurt Salk deeply, and he retreated into the
seclusion of his laboratory at a time when the public wanted to give him a tickertape
parade.
In 1955, a commercial laboratory carelessly produced and sold batches of Salk vaccine
containing unkilled viruses. The defective vaccine caused 204 polio cases with 11deaths
and over 100 cases of paralysis. Albert Sabin and the establishment rallied with an attack
of vigor and by 1956, the medical establishment had produced a weakened, live virus
vaccine (the Sabin vaccine). In 1964, the Surgeon General stated there was only a small
risk involved with the Sabin vaccine. He failed to mention that with the killed Salk
vaccine, properly prepared, there was no risk at all. The Salk vaccine became passÚ.
Today, the Sabin vaccine is preferred by physicians.
H
UMOR: ON THE FUN SIDE
- Word Search (see Appendix for master copy)
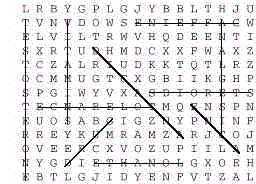
Words about the concepts in this module can be obtained from the clues given. Find
these words in the block of letters:
- Substance that reacts with excess stomach acid.
- Common name for a substance that changes a physical or psychological function in the
body.
- Need of increasing amounts of a substance to experience the same initial effects.
- Acetaminophen, ibuprofen, or acetylsalicylic acid, for example.
- Benzoyl peroxide is the active antiseptic ingredient in ____ ointments.
- These organic compounds are required in small amounts in the diet.
- Stimulant in cola and coffee.
- Birth control drugs belong to this class of organic compounds.
- The amount of this drug is determined in the Breathalyzer test.
- Anabolic steroids are synthetic derivatives of this natural compound.
Answers:
- ANTACID
- DRUG
- TOLERANCE
- ANALGESIC
- ACNE
- VITAMINS
- CAFFEINE
- STEROIDS
- ETHANOL
- TESTOSTERONE
2. See cartoons at end of module.
MEDIA
- The CHEM Study film "Molecular Structure and Health." Time-lapse
photomicrography of laboratory demonstrations helps to identify the role of molecular
structure in determining biological activity. Students will also see how the correlation
of the structure and biological activity of sulfanilamide with a vitamin essential for
bacterial growth leads to a more general presentation of the biochemical nature of growth.
Color, 22 min. Available from Ward’s Natural Science Establishment, Inc., 5100 West
Henrietta Road, P.O. Box 92912, Rochester, NY 14692-9012, (716) 359-2509, (800) 962-2660;
(716) 334-6174 FAX.
- Software published by Project SERAPHIM, Department of Chemistry, University of
Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706-1396: (608) 263-2837 (voice)
or (608) 262-0381 (FAX).
a. For the Apple II computer running on ProDOS: AR 702
b. For the Apple II computer: AP 701
- Videodisc published by JCE: Software, a publication of the Journal of Chemical
Education, Department of Chemistry, University of Wisconsin-Madison, 1101 University
Avenue, Madison, WI 53706-1396: (608) 262-5153 (voice) or (608) 262-0381 (FAX).
"From an Amino Acid to a Peptide Chain," "The Alpha Helix," and
"DNA Structure, Synthesis of Messenger RNA, Protein Synthesis," three chapters
on The
World of Chemistry: Selected Demonstrations and Animations : Disc II (double sided, 60
min.), Special Issue 4.

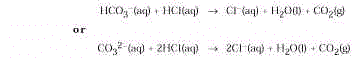



















![]() Drug Listing page for
the above mentioned informaiton *
Drug Listing page for
the above mentioned informaiton *