

Drinking Water
When considering the topic of solubility and solutions, one inevitably must discuss the role of water as the ‘universal’ solvent. While this property is necessary to sustain life, impurities can be dissolved or suspended in water and are often dangerous or even fatal to humans. The earliest records of water treatment and purification have been traced to approximately 2000 B.C. in the Sus’ruta Samhita, a body of Indian Sanskrit medical knowledge. Although not written down until 400 A.D., this oral tradition described a process to purify water by boiling over a fire, or being heated in the sun, or by dipping a heated iron into it; another way was to purify by filtration through sand and coarse gravel. During the period from the 15th to 13th centuries B.C. the Egyptians developed an apparatus for filtering and siphoning fluids and recorded images of them on the walls of the tombs of Amenophis II and Ramses II. By the eighth century A.D., Arabian alchemists had developed methods of distillation that provided a safer product than that of filtration.
Most of Europe did not begin to recognize the need for water purification until the end of the Middle Ages. Sir Francis Bacon conducted numerous experiments on filtration, boiling, distillation, and clarification by coagulation. In 1685 an illustrated description of sand filters was published by Lucas Antonius Portius, an Italian physician. Portius had participated in efforts at mass sanitation for soldiers fighting the Austro-Turkish War of 1685. He proposed straining and sedimentation followed by multiple filtration through sand. Nevertheless, most Europeans continued to get their drinking water from streams, rivers, and rainwater collecting bins. They then brought these simple methods to the new world with them.
Until the end of the 19th century, Americans obtained drinking water from local ponds, wells, and rainwater holding tanks. Waste water was discarded in dry wells, leaching cesspools (pits lined with broken stones), or was simply dumped on the ground. Human defecation was usually collected in receptacles lined with brick or stone that were periodically emptied. However by the mid 1880s, flush toilets were beginning to find common use in urban settings. As a result, municipal sewer systems were constructed to carry the sewage. Until 1909 these sewer wastes were released without treatment into streams and lakes from which either the same urban center or others drew their drinking water. It was believed that rivers and large bodies of water would purify themselves.
As a result of disease epidemics that were striking urban centers at the turn of the century through the 1920s, urban centers began treating both the raw sewage from household wastes as well as chlorinating drinking water. Thus water is cleaned twice, once before being used, and again after use. The before-use cleaning, known as water treatment, takes place at municipal treatment plants. After its use, municipal water must again be treated, this time at a sewage treatment plant.
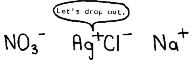
Word Search (see Appendix for master
copy)
Words about the concepts in this module can be obtained
from the clues given. Find these words in the block of
letters:
1. Ability of a substance to dissolve in a given quantity
of solvent
2. Solid formed by reaction in aqueous solution.
3. Color of solid formed when solutions of silver nitrate
and sodium chloride are mixed.
4. Color of solid formed when solutions of lead nitrate
and potassium iodide are mixed.
5. Ionic solids dissolve in this kind of solvent.
6. Solubility of most solids increases as this factor is
increased.
7. Symbol for solubility product constant.
8. For PbI2 Ksp = [Pb2+]x
[I -] y . The sum of x + y is this
number.
9. Compounds containing this polyatomic cation are
soluble.
10. Halides of this metal are used in photography.
Answers: 1. SOLUBILITY 2. PRECIPITATE 3. WHITE 4. YELLOW
5. POLAR 6. TEMPERATURE 7. KSP 8. THREE 9. AMMONIUM 10.
SILVER}
See cartoons at end of module.
(page 17 & 18)