Return to Table of Contents
 Activity 1: Classifying Substances Based on Their Reactions.
Activity 1: Classifying Substances Based on Their Reactions.
Introduction
Chemists find it convenient to classify the overwhelming number of known substances into categories that share common properties. In this laboratory activity you will become familiar with some chemical behaviors of certain types of substances that allow them to be classified into acids, bases, and salts.
Purpose
To develop a scheme for classifying different substances as acids,
bases, or salts.
Safety
1. Wear protective goggles and apron throughout the laboratory
activity.
2. The acids and bases used are corrosive. Follow your teacher's instructions in handling acids and bases and for waste disposal. All spills should be cleaned up immediately.
3 Accidental spills on the body should be flushed with water; notify your
teacher.
4. Barium hydroxide is toxic.
5. Dispose of the chemicals as your teacher directs.
6. Thoroughly wash your hands before leaving the laboratory.
Part I Procedure
1. Arrange a matrix within the well plate or among the test tubes to match
the pattern shown in Figure 7.
2. Add a drop of phenolphthalein to a sample of each of the seven test
solutions. Record your observations.
3. Add a drop of bromthymol blue to a different sample of each of the seven
test solutions. Record your observations.
4. Add a drop of universal indicator to a different sample of each of the
seven test solutions. Record your observations.
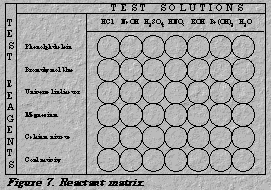 5. Clean a piece of magnesium ribbon with fine-grade sandpaper or steel wool until it is shiny. Cut the ribbon into seven pieces small enough to fit in the test tubes or wells.
5. Clean a piece of magnesium ribbon with fine-grade sandpaper or steel wool until it is shiny. Cut the ribbon into seven pieces small enough to fit in the test tubes or wells.
6. Add a piece of magnesium, Mg, to each of the seven test solutions. Record
your observations.
7. Add a drop of calcium nitrate solution, Ca(NO3)2(aq), to a different
sample of each of the seven test solutions. Record your observations.
8. Use the conductivity tester on the last sample of each of the seven test
solutions. Rinse the conductivity tester in fresh distilled water after testing
each sample. Record your observations.
Acids and Bases
(Page 7)
 Activity 1: Classifying Substances Based on Their Reactions.
Activity 1: Classifying Substances Based on Their Reactions.
 5. Clean a piece of magnesium ribbon with fine-grade sandpaper or steel wool until it is shiny. Cut the ribbon into seven pieces small enough to fit in the test tubes or wells.
5. Clean a piece of magnesium ribbon with fine-grade sandpaper or steel wool until it is shiny. Cut the ribbon into seven pieces small enough to fit in the test tubes or wells.
