Language of Chemistry
-
Brønsted-Lowry acid - a proton donor.
- The word acid comes from the Latin acidus, for sour. One property of
acids is their sour taste, such as that experienced with yogurt, pickles, and
lemons.
- The terms strong and weak are not synonymous with concentrated and dilute, respectively (see Common Misconceptions).
- For consecutive integer values of pH, H+ concentrations differ by
a factor of 10. For example, solutions of pH = 1 ([H+] = 10-1
M) and pH = 2 ([H+] = 10-2 M) have a H+
concentration ratio of 10, that is, 10-1 / 10-2 = 10
- Solutions with high hydrogen ion concentration have a low pH. A solution
with pH = 1 has a [H+] = 10-1 M; a solution with pH = 6
has a [H+] = 10-6 M. Figure 17 provides convenient
conversion scales between pH and concentration.
- pH-pOH Square. Equations over or next to arrows are conversion equations. Given one corner, the other can be calculated using the conversion equation.
Brønsted-Lowry base - a proton acceptor.
Concentration - the number of moles solute (for example, acid or base) per liter of solution.
End point - the point in a titration when the indicator changes color.
Indicator - a weak acid or base which changes colors over a narrow pH range.
Lewis acid - an electron acceptor.
Lewis base - an electron donor.
Molarity - a concentration term defined as the number of moles of solute per liter of solution.
Neutralization - a reaction between an acid and a base in which the acid and base properties disappear.
Strength - the percentage of ionized molecules of acid (or base).
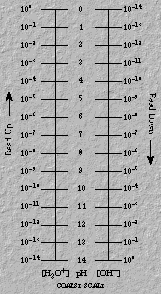

Figure 17. Scales for pH-concentration relationships.
.
.
.
Figure 18. pH-pOH square.
(Page 23)
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|