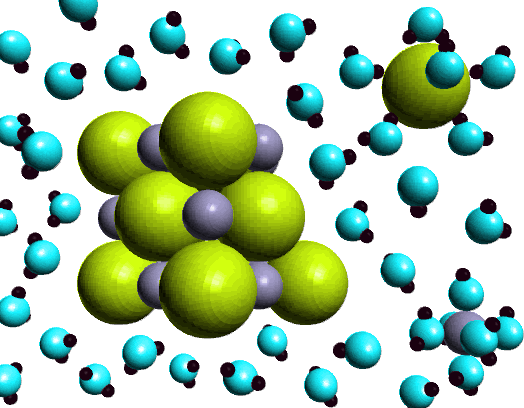
 |
In this figure there are two examples of ion-dipoe attractive forces. Notice the water molecules surrounding the chloride (large green sphere) and the water molecules surrounding the sodium ion (smaller gray sphere). In particular notice the orientation of the water molecules. Chloride ion has a neqative charge in NaCl, and water is a polar molecule with a permanent dipole. Since water has a dipole there is a region of partial negative charge, on the oxygen atom, and regions of partial positive charge, on the hydrogen atoms. The water molecule is oriented so the hydrogen atoms, with the partial positive charge, are close to the negatively charged chloride ion. In the case of the positively charged sodium ion, the oxygen atoms are aligned towards ion. The interaction between the chloride ion, and the sodium ion and the water molecules is an example of ion-dipole interaction. |