LEWIS ELECTRON–DOT STRUCTURES
Lewis structures will be our first step in describing several
models which will help us organize a large amount of experimental
data. We can apply our knowledge of the electronegativity and the
octet rule to extent our ability to write more complicated Lewis
electron–dot formulas. Practice writing Lewis formulas will
help us understand many of the ratios of elements, bonding
geometry and polarity and intermolecular attractive forces that
occur in covalent compounds. The object will be to predict a
reasonable Lewis electron–dot structure given the chemical
formula. To do this we must follow some rules which are stated in your text
(Page 269).
Write the skeletal structure for the molecule.
most electronegative atoms are terminal (O,
F, Cl)
molecules with symmetrical formulas may have
symmetrical structures
Determine the total number of valence electrons by
adding the valence electrons for all atoms. For anions,
add an amount equal to the charge of the anion to the
total number of valence electrons. For cations, subtract
an amount equal to the charge of the cation from the
total number of valence electrons.
Count up the electrons required to build the skeletal
structure in part 1), and subtract from the number of
electrons in part 2).
-
Distribute the remaining electrons so each terminal atom atom has
an octet of electrons. Any remaining electrons go to the central atom. Note: it maybe necessary to
involve multiple bonds.
We will discuss some examples to demonstrate the procedure
for writing correct Lewis electron–dot structures.
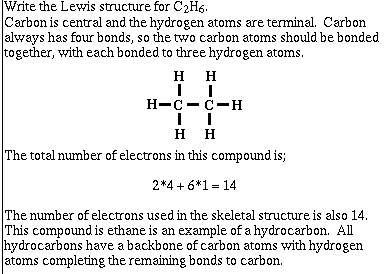
Example #1
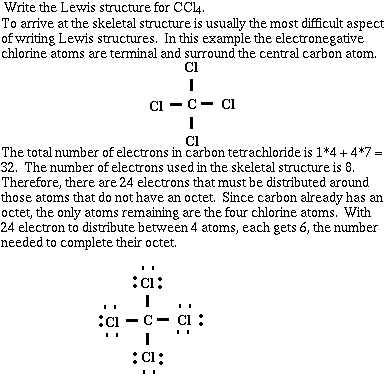
Example #2

Example #3;
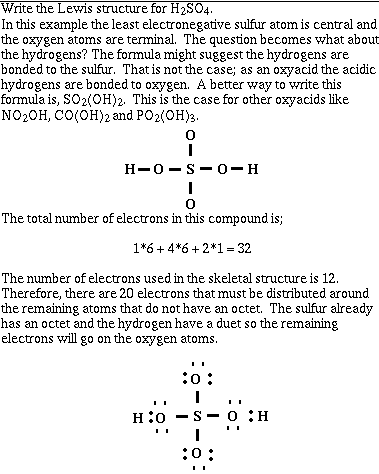
Example #4;
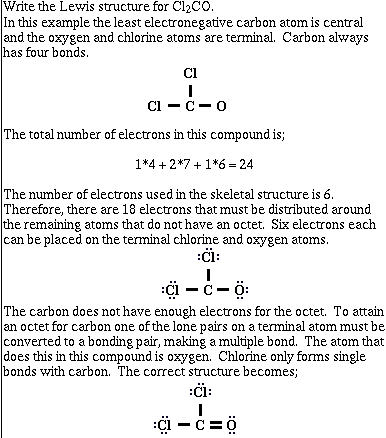
Multiple bonds are formed when two atoms in a chemical bond
share more than one pair of electrons. In the example above 2
pairs of electrons were placed between the carbon and the oxygen.
This is referred to as a double bond. A double bond is stronger
than a single bond. The Lewis structure of the molecule CO
demonstrates another type of multiple bond. The correct Lewis
structure for carbon monoxide has three pairs of electrons
between the carbon and oxygen. Such an arrangement is called a
triple bond. This is stronger than a carbon oxygen double bond.
The C–O bond distance in the series CH3OH
(C–O is 1.43 Å), Cl2CO (C–O is 1.22 Å) and
CO (C–O is 1.13 Å). The more electrons between the atoms
the short the distance between the atoms and the stronger the
bond.
Sample Problem #5;