A titration is performed using by adding 0.100 M NaOH to 40.0 mL of 0.1 M
HA (Ka is 1.0 x 10-5.).
b') Calculate the pH after addition of 20.0 mL of 0.100 M NaOH.
Answer:
When a strong base like NaOH is added to a weak acid like HA a neutralization
reaction occurs,
NaOH(aq) + HA(aq) ---> NaA(aq) + H2O(aq)
The K for this reaction is very large,
so the reaction goes to completion. To determine how much reaction occurs we
need to set up an ICE 'like' table.
|
NaOH(aq)
|
+ HA(aq)
|
--->
|
NaA(aq)
|
+ H2O(aq)
|
I
|
|
|
|
|
|
C
|
|
|
|
|
|
F
|
|
|
|
|
|
To begin filling out the table above we need to know the initial number of
moles of all species in the chemical equation.
The moles of NaOH added from the buret are;
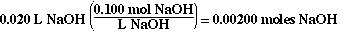
The moles of HA in the flask initially are;
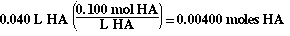
So the 'ICE table' now looks like;
|
NaOH(aq)
|
+ HA(aq)
|
--->
|
NaA(aq)
|
+ H2O(aq)
|
I
|
0.00200 mol
|
0.00400 mol
|
|
0
|
-
|
C
|
|
|
|
|
|
F
|
|
|
|
|
|
Since K for the reaction is large, the reaction will go to
completion. This means that the reactant in the smallest amount will completely
react. At this point in the titration there are fewer moles of base compared
to acid. So all the base reacts. Adding this to the 'ICE table' we have;
|
NaOH(aq)
|
+ HA(aq)
|
--->
|
NaA(aq)
|
+ H2O(aq)
|
I
|
0.00200 mol
|
0.00400 mol
|
|
0
|
-
|
C
|
-0.00200 mol
|
-0.00200 mol
|
|
+0.00200 mol
|
-
|
F
|
|
|
|
|
|
The final amount of each species can be obtained just as it
always has been in the other ICE tables, by adding the change to the initial
amount.
|
NaOH(aq)
|
+ HA(aq)
|
--->
|
NaA(aq)
|
+ H2O(aq)
|
I
|
0.00200 mol
|
0.00400 mol
|
|
0
|
-
|
C
|
-0.00200 mol
|
-0.00200 mol
|
|
+0.00200 mol
|
-
|
F
|
0
|
0.00200 mol
|
|
+0.00200 mol
|
|
Focusing on the Final row there is acid remaining. All the
base has reacted and the salt of the weak acid and strong base is formed.
To calculate the pH we must recognize the type of system we
have. To do that we look at the final row and see what species are present.
In this case there are moles of a weak acid (HA) and its salt (NaA). This
is a common ion system, one with a weak acid and its conjugate base (salt).
To calculate the pH of the solution we must set up the ICE
table for the common ion. In the ICE table we must express the concentration
of HA and A-. From the ICF table above the moles of HA and A-
are given. The concentration can be determined by dividing the moles of HA
and A- by the volume of solution.
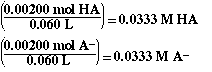
Substituting,
|
HA(aq)
|
--->
|
H+(aq)
|
A-(aq)
|
I
|
0.0333 M
|
|
~ 0
|
0.0333
|
C
|
- x
|
|
+ x
|
+ x
|
F
|
0.0333 - x
|
|
+ x
|
0.0333 + x
|
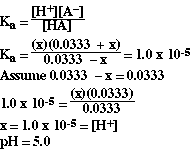
We can see this point on the titration curve (as point 2b').

This is a particularly interesting point on the titration curve
for a weak acid and a strong base. Reviewing the ICE table above you should
have noticed that at this particular volume the moles of HA = moles of A-.
Looking at the pH calculation we notice the concentration of HA and A-
cancel because they are equal. At this point in the titration the [H+]
is equal to the Ka for the weak acid. This is called the half-equivalence
point. At this point in the titration the pH = pK for the weak acid.