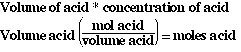This table is ICE 'like' because is differs in some respects
from a normal ICE table
|
NaOH(aq)
|
+ HA(aq)
|
--->
|
NaA(aq)
|
+ H2O(aq)
|
I
|
|
|
|
|
|
C
|
|
|
|
|
|
F
|
|
|
|
|
|
The three rows are labeled I (initial), C (change) and F (final). Instead
of ICE, I have used F for final because unlike the K's for reactions that we've
used ICE tables, the K for this reaction is very large. With such a large K
the reaction goes completely to the right.
The entries in this table are always in terms of moles, not concentrations
like our normal ICE table were.
So to complete the table we must determine the moles of base and acid.
Since we know the volume of the acid or base and its concentration we can
determine moles;
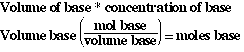
or