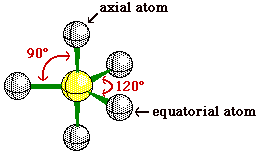
When the central atom has 5 bonding pairs of
electrons and no non-bonding pairs the geometry is trigonal bipyramidal. Notice those
atoms in the trigonal plane are referred to as equitorial
atoms, while the atoms perpendicular to the trigonal
plane are called axial atoms.
|
 |
If a bonding pair of electrons is replaced with a
nonbonding pair the geometry is described as a seesaw shape. The nonbonding pair
of electrons is always located in the equatorial position
never the terminal position to minimize electron-pair
repulsions.
|
 |
When the central atom has 3 bonding pairs of
electrons and 2 nonbonding pair the geometry is T–shaped. Again the two
nonbonding pairs of electrons are located in equatorial
positions.
|
 |
When the central atom has 2 bonding pairs of
electrons and 3 nonbonding pair the geometry is linear.
|
 |