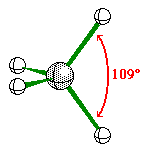
When the central atom has 4 bonding pairs of
electron the geometry is tetrahedral.
All of the bond angles are 109.5Ü.
|
|
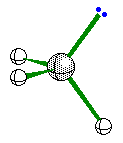
When the central atom has 3 bonding pairs of
electrons and 1 nonbonding pair the geometry is trigonal pyramidal.
|
|
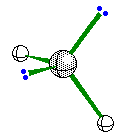
When the central atom has 2 bonding pairs of
electrons and 2 nonbonding pair the geometry is bent.
|
|
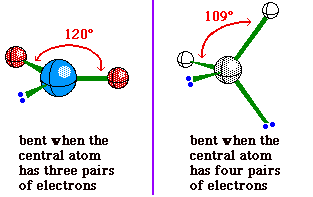
Although bent is used to describe the molecular
geometry for a molecule with a central atom having 2
bonding pairs of electrons and 2 non-bonding pairs, it is
also used to describe the molecular geometry for a
molecule with a central atom having 2 bonding pairs of
electrons and 1 nonbonding pair. The difference is in the
bond angle.
|
 |