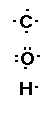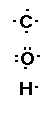To draw the Lewis structure of C2H6O we
first need to determine the number of valence electrons around
carbon, hydrogen and oxygen.
How many valence electrons around carbon, hydrogen and
oxygen?
Answer
Carbon has 4 valence electrons, oxygen 6 valence electrons
and hydrogen has 1 valence electrons.
We can write the Lewis structure for the atoms showing the
valence electrons as,
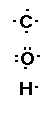
To draw the Lewis structure we now try arranging
the two carbon atoms, six hydrogen atoms and one oxygen atom so
both carbon and oxygen have/share eight electrons and hydrogen
has/shares two electrons.
Try drawing the two possible structures on your
own. Answer