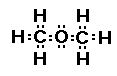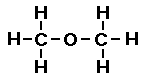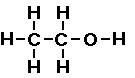Here is how I would approach the problem of drawing two
structures for the compound C2H6O.
Given the Lewis structures of the atoms
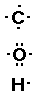
and the formula C2H6O, I
would recall that in organic compounds there are always methyl
groups, CH3-. Since there are two carbon atoms and six
hydrogen atoms I can make two methyl groups,
CH3- and CH3- or 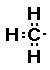 and
and 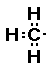
So now I have two methyl groups and one oxygen
atom.
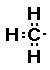 and
and 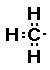 and
and 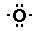
The oxygen atom wants to form two bonds (it has
two unpaired electrons) and each methyl group wants to form one
bond (it has one unpaired electron).

So we just bring the two methyl groups in on
either side of the oxygen atom and form the bonds.
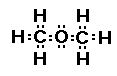
We can also represent this structure as,
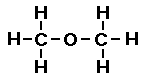
If we write this structure using condensed
formula it would look like, CH3OCH3.
The oxygen atom in this structure is bonded to two carbon atoms. When oxygen is bonded to
two carbon atoms the compound type is called an ether. We say the
R-O-R arrangement is an ether functional group. Here R- is the
abbreviation for an organic group with an available carbon bond.
Well that is one structure...what is the
other??? The other structure must use the same atoms, but the
arrangement of the atoms is different. How could we change the
arrangement? In this arrangement we have the oxygen atom between
the two carbon atoms. The other arrangement we will move the
oxygen between a carbon and a hydrogen atom. So it would look
like,
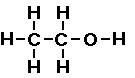
It does not make any difference which carbon and
hydrogen we choose they are all the same. This is a different
arrangement of the same atoms. In this case the oxygen atom is
bonded to one carbon atom and one hydrogen atom. This compound is
called an alcohol. We say the R-O-H arrangement is an alcohol
functional group. If we write this as a condensed formula is
looks like C2H5OH or CH3CH2OH.
We now know two important functional groups,
ether and alcohol.
![]()
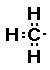 and
and 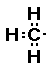
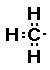 and
and 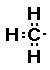 and
and