Answer
Can you draw the structure of dimethylamine?
Ball-and-Stick
|
Lewis Structure
|
Electron-dot Structure
|
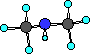
|
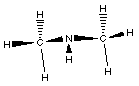
|
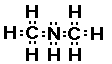
|
This compound has a nitrogen atom with three bonds; two to
carbon atoms and one to a hydrogen atom.
We could continue by drawing the structure of trimethylamine,
or ethylamine as additional examples of amines.
But I want you to be able to look at a structure and find the
amine functional group.
So lets look at some more disgusting examples of amines to
test your ability to find the amine functional group.
Have you ever smelled putrescine or cadaverine? These amines
are found in urine and bad breath. How would you know these are
amines, well not from the names, but by finding the functional
group given the structure. Here are the structures of these two
very smelly compounds.
Compound
|
Ball-and-Stick
|
Lewis Structure
|
Electron-dot Structure
|
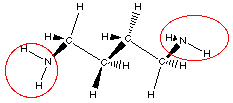
putrescine
|
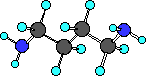
|
|
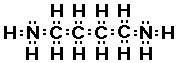
|
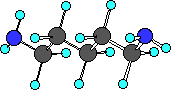
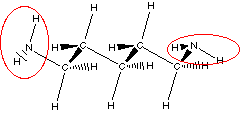
cadaverine
|
|
|
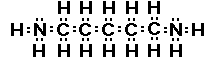
|
Do you see there are actually two amine groups in putrescine
and in cadaverine.
So we can recognize amine functional group by locating a
nitrogen atom with three bonds one of which is to a carbon atom.
We can use the notation R- as a carbon containing substituent. So
the amine functional group is RNH2, or R2NH,
or R3N.
Return
to man functional group page.