GROUP AND DISCUSSION ACTIVITIES
 Key Questions Key Questions |
 Language of Chemistry Language of Chemistry |
 Counterintuitive
Examples Counterintuitive
Examples |
 Problem
Solving Problem
Solving |
 Analogies and
Metaphors Analogies and
Metaphors |
 Humor:
On The Fun Side Humor:
On The Fun Side |
 Pictures in
the Mind Pictures in
the Mind |
 Media Media |
Key Questions
- What happens on a particle level when crystals form
from evaporation of a saturated solution? [As water slowly evaporates, the ions deposit
on the crystal because the saturated
solution cannot dissolve more solute.]
- How can flame tests and borax bead tests be used in mineral identification? What
are the limitations of these tests? [Both flame tests and borax bead tests are used to identify the positive ion of the
mineral. Sometimes the colors are very similar and these tests cannot identify the
negative ion.]
- What is the basic unit common to all silicate minerals? What is its geometry? [The SiO 4 4– ion. It is tetrahedral.]
- How are crystals formed from a supersaturated solution? [A single crystal is added to disturb the
solution and then many crystals form rapidly.]
- What factors are characteristic of minerals that are classified as gemstones? [Color, transparency, and luster or
brilliance.]
- Compounds of the transition elements often exhibit color. To what can this color be
attributed? [If the transition element forms a compound in which its d orbitals do not all have the same energy, the
result is an energy gap that matches the energy of photons of visible light. When white
light strikes the compounds, those photons are absorbed. The color we see is the result of
the colors that are not absorbed.] (See Transition Elements module.)
- How is a gemstone different from a mineral? [A gemstone, generally, forms when a particular transition element atom is
incorporated as an impurity in a parent mineral. For example, ruby results from Cr 3+ ions replacing some of the Al 3+ ions in the mineral corundum, Al 2 O 3 .]
Counterintuitive Examples
Salol (phenyl salicylate), a solid at room
temperature, is a substance that supercools readily. Supercooling is the process of
cooling a liquid below its freezing point without changing to a solid. Prepare microscope
slides that have two "globs" of salol as shown here. Have students melt the
larger glob by holding a match under it. After melting the glob place the slide on the
desk top to allow it to cool to room temperature. Ask how the solid and liquid can
both exist at the same temper- ature. Then use a spatula or stirring rod to break off a
crystal from the smaller glob and move it to the puddle formed from the larger glob. The
slow crystallization holds the interest of students.

Analogies and Metaphors
Fruit is frequently stacked in supermarkets
in a pattern analogous to cubic close packing.
Pictures in the Mind
Making Models of Silicate Minerals
Introduction
Students sometimes have great difficulty visualizing
in three dimensions. This activity gives them hands-on experience imagining various
silicate ions. Commercial chemistry models can be used by declaring that the ball that
usually represents the carbon atom represents a silicon atom. However, gum drops and tooth
picks are better because students will have to decide the appropriate angles.
Materials
Model kits or gum drops of two colors and tooth
picks.
Procedure
- Require students to work in groups of two or three.
- Explain that silicon has four covalent bonds and the bonds
need to be as far apart as possible. Remind them to consider three dimensions.
- Direct students to make an SiO 4 4– ion, and draw a projection on paper.
Circulate through the room correcting those insisting on using only two dimensions.
- Make models and drawings of:
- Si 2 O 7 6–
chains of (SiO 3 2– ) n and double chains of (Si
4 O 11 6– ) n
- planar sheet of atoms where each Si atom shares oxygen atoms with three other silicons
- a three-dimensional structure of empirical formula SiO 2 .
- Have students describe the properties of minerals that would
have each of the structures they made. Tips
for the Teacher and the Transparency Masters will
be useful in interpreting students’ drawings and providing examples. If samples of
minerals with each type of structure—chains, sheets, and three-dimensional—are
available, they will add to the understanding of the relationship between structure and
properties.
Language of Chemistry
crystal substance in which
the atoms, ions, or molecules are arranged in an orderly, repeating three-dimensional
pattern called a crystal lattice.
lithosphere the upper portion of the earth’s crust.
mineral inorganic solid of definite composition found in the
earth’s crust.
silicates minerals with crystal structure containing silicon-oxygen
tetrahedra.
supercooling process of cooling a liquid below its freezing point
without changing to a solid.
Silica and silicates are compounds made primarily of
silicon and oxygen atoms.
They can be discussed to make three important points:
- Many minerals are silicate based.
- Lewis structure pictures can be used to classify minerals.
- Lewis structure pictures can help explain physical properties of minerals.
All silicate minerals contain Si atoms bonded to four O atoms in a tetrahedral
arrangement. The simplest involve the orthosilicate ion (SiO 4
4– ).

Each oxygen is bonded to only one Si and has a residual negative charge. Examples of
orthosilicate minerals are zircon (ZrSiO 4 ) and
forsterite (Mg 2 SiO 4 ).
When an oxygen atom is shared by two Si atoms, the pyrosilicate anion (Si 2 O 7 6– ) is
obtained.

The Lewis structure shows single bonds, octets on each atom, and a tetrahedral geometry
around the Si atom. Some pyrosilicate minerals are akermanite (Ca 2 MgSi 2 O 7
), lawsonite (CaAl 2 Si 2 O 7 (OH)2
), and hemimorphite (Zn 4 (OH)2 Si 2 O 7 ).
The silicate units can continue to link through sharing oxygen atoms to form long
single chains of the general formula SiO 3 2–

or long double chains having the formula Si 4 O 11 6– .

Examples of some of SiO 3 2– minerals are
enstatite(MgSiO 3 ) and spodumene (LiAl(SiO 3 ) 2 ). Tremolite (Ca 2 Mg 5 Si 8 O 22 (OH)2 ) is an example of a mineral with double chains. SiO x tetrahedra can also link into two-dimensional planar sheets
of atoms, where each Si atom shares oxygen atoms with three other silicons. These minerals
have the general formula Si 4 O 10 4– and are called sheet silicates. Chrysolite (Mg 6 Si 4 O 10 (OH)8 ) is an example.
In micas one Si is replaced by an Al, for example, muscovite (KAl 2 AlSi 3 O 10
(OH)2 ).

A logical extension of this build-up of tetrahedra is to have all four oxygens in a
tetrahedral silicate unit shared by Si atoms in adjacent units. The result is quartz, with
an empirical formula of SiO 2 . The quartz structure
can be imagined by viewing the three-dimensional linked structure of diamond. SiO 2 would have Si’s where each C is in the diamond
structure; the oxygen atoms are then midway between each pair of Si atoms.
The one-, two-, or three-dimensional links of SiO x units
lead to their physical properties. Asbestos is mixed minerals of the long, double-chain
type. Thus, they are needle-like, which may account for some of the damage they do to
living tissue. Micas are slippery due to two-dimensional sheets of SiO x units that can slide over each other. Quartz has structural strength
because of its three-dimensional covalent linking of SiO x units.
Problem Solving
Many common minerals contain oxygen as a major
component. Some of these are easily collected and identified. This problem-solving
activity shows the variation of that oxygen content. Problem: Calculate the oxygen content
of flint or quartz (SiO 2 ).
Find the molar mass of the compound:

The part of the molar mass due to oxygen can be calculated as a percentage of the
whole:
Sample Problems: Calculate the percent oxygen in the minerals:
- Calcite, CaCO 3 [48.0%]
- Corundum, Al 2 O 3 [47.1%]
- Gypsum, CaSO 4 ·2H 2 O
[59.2%]
- Hematite, Fe 2 O 3 [30.1%]
- Orthoclase (K-feldspar), K(AlSi 3 O 8 ) [50.1%]
HUMOR: ON
THE FUN SIDE
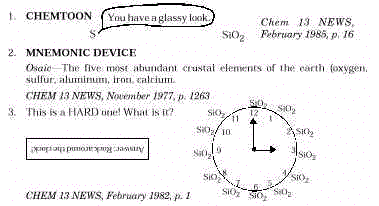
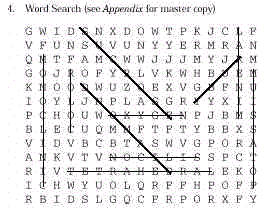
Words about concepts in this module can be obtained from the clues given.
Find these words in the block of letters:
- Silicates are compounds made primarily of oxygen and this element.
- A gem whose basic mineral is Al 2 O 3 with Cr(III) as an impurity.
- This property of gemstones is characterized by presence of transition elements as
impurities.
- This gemstone is an allotrope of carbon.
- Most abundant element in the earth’s crust, by mass.
- Inorganic solid of definite composition found in the crust.
- Silicates have this geometrical arrangement.
- Rock candy is this organic compound.
- One of the six basic crystal systems.
- A lavender flame test identifies this element.
Answers:
- SILICON
- RUBY
- COLOR
- DIAMOND
- OXYGEN
- MINERAL
- TETRAHEDRAL
- SUCROSE
- MONOCLINIC
- POTASSIUM
5. See cartoons at end of module.
MEDIA
- The World of Chemistry (high school version)
videotapes. WINGS for Learning/SUNBURST, 101 Castleton Street, Pleasantville, NY 10570;
(800) 321-7511; (914) 747-3310; (914) 747-4109 (FAX).
- "The Chemistry of Earth": reinforcing chemistry topics through examples from
geology—solubility, precipitation, equilibrium, and acid-base chemistry applied in
explaining mineral deposition and world-wide distribution of ores and silicate
minerals; the relationship between chemical structure and macroscopic properties—cave
formation and stalactite and stalagmite development; limestone.
- "The Atmosphere": Common theory on formation of the atmosphere; the
composition today—highlights of some environmental concerns: contaminants being added
to the atmosphere, the greenhouse effect, the hole in the ozone layer, the commercial use
of CFC’s.
- "Chemical Bonds": Ionic and covalent bonds defined through graphic
illustration — a comparison of their relative strength in ionic crystals (sodium
chloride) and network covalent solids (diamond)—molecular solids; the difference
between the strong intramolecular covalent bonds between atoms in a molecule and the
weaker intermolecular attractions, such as hydrogen bonds, between molecules.
- MECC (Minnesota Educational Computer Consortium, St. Paul, MN 55165):
"Murphy’s Minerals," a computer game to practice skills learned in
identifying minerals. (612) 569-1500.Planet Earth Series: A seven-part video series that
was first shown on the Public Broadcasting Service in 1986. This series is a rich and
exciting investigation of the earth sciences today, a vivid panorama of the planet we call
home. Produced by WQED/Pittsburgh, in association with the National Academy of Sciences,
this series includes the episodes: "The Living Machine," "The Blue
Planet," "The Climate Puzzle," "Tales from Other Worlds,"
"Gifts from the Earth," "The Solar Sea," and "Fate of the
Earth."
The episode most
useful with this topic is "Gifts from the Earth." This program acquaints
students with the breadth and variety of the world’s natural resources
through a story
spanning millions of years. Included are segments explaining how mineral-saturated
hydrothermal vents on the sea floor ultimately became gold
and copper mines
on land; about the slow accumulation of fossil fuels; of the ingenious scientists who seek
out the secrets of the earth. The formation of metallic
elements,
minerals—both precious and common—are explored, as well as how these resources
influence civilization. One of the handouts, "Minerals in Our
Lives," is
particularly interesting as it lists 60 common elements and compounds readily
available from the earth’s crust and describes uses of each. Available
from: PBS Video,
1320 Braddock Place, Alexandria, VA 22314. Telephone: (703) 739-5000.
The episode
"The Blue Planet" deals primarily with the influence of liquid water on the
surface of the earth, and could also be used in this module.
- "Gemstones of America," STS Film & Video Productions, P.O. Box 27477, Salt
Lake City, UT 84127. A Smithsonian Project, 1991. (801) 263-3959.
- Software published by JCE: Software, a publication of the Journal of Chemical
Education, Department of Chemistry, University of Wisconsin-Madison, 1101 University
Avenue, Madison, WI 53706-1396: (608) 262-5153 (voice) or (608) 262-0381 (FAX).
- KC? Discoverer with Knowledgeable Counselor, by Daniel Cabrol, John W. Moore and
Robert C. Rittenhouse. Special Issue 2, for IBM PS/2, PC compatible computers.
- KC? Discoverer: Exploring the Properties of the Chemical Elements, by Aw Feng and
John W. Moore. Vol. I B, No. 1, for IBM PS/2, PC compatible computers.
- KC? Discoverer?, by Michael Liebl, Vol. IV A, No. 2, for all Apple II computers.
- The Periodic Table Stack, by Michael Farris. Vol. I C, No. 1, for the Apple
Macintosh.
- Software published by Project SERAPHIM, Department of Chemistry, University of
Wisconsin-Madison, 1101 University Avenue, Madison, WI 53706-1396: (608) 263-2837 (voice)
or (608) 262-0381 (FAX).
- For the Apple II computer: AP 807
- For IBM PS/2 PC-compatible computers: PC 3702
- Videodiscs published byJCE: Software, a publication of the Journal of Chemical
Education, Department of Chemistry, University of Wisconsin-Madison, 1101 University
Avenue, Madison, WI 53706-1396: (608) 262-5153 (voice) or (608) 262-0381 (FAX).
- "Earth’s Atmosphere," "Acid Rain and Limestone" and
"Silicates," three chapters on The World of Chemistry: Selected
Demonstrations and Animations: Disc II (double sided, 60 min.), Special Issue 4.
- The Periodic Table Videodisc (single side, 30 min.). Special Issue 1.
- Gems and Minerals: The Ultimate Rock Video, available from Smithsonian Laserdisc
Collection, Smithsonian Institution, Washington, DC; (202) 357-1300.






![]()

