DEMONSTRATIONS
 Demonstration 1: Growing Crystals in Gels
Demonstration 1: Growing Crystals in Gels |
 Demonstration 2:
An Inorganic Polymerization Demonstration Demonstration 2:
An Inorganic Polymerization Demonstration |
 Demonstration 3:
Supersaturated Solution Demonstration 3:
Supersaturated Solution |
 Demonstration 4:
Precipitation and Redissolution of Calcium Carbonate Demonstration 4:
Precipitation and Redissolution of Calcium Carbonate |
 Demonstration 5:
Pot-O-Gold Demonstration 5:
Pot-O-Gold |
CAUTION: Use appropriate safety guidelines in performing demonstrations.
Demonstration 1: Growing Crystals in
Gels
Description
In nature minerals are often found crystallized in bands. Large, nearly perfect
specimens are occasionally found. These phenomena can be simulated by preparing a gel from
water glass (a solution of Na
2 SiO3 ) to represent the silicate rock. Small quantities of other
compounds are introduced, and crystals are formed by precipitation or oxidation reduction
reactions. A solution of one of the ions of the desired crystal is added to an acidified
solution of water glass. This mixture is allowed to stand. When the other reactant is
added to the top of the solution the ions diffuse downward, and the reaction takes place.
The longer these crystals are permitted to grow, the more beautiful they become.
Materials
- Beaker, 400-mL
- 4 Test tube, 16- x 150-mm
- 4 Stoppers
- ParafilmÔ
- 1.0 M Copper(II) chloride, CuCl
2 , 100 mL (17.0g CuCl2
. 2H2O per 100 mL solution)
1.0 M Lead acetate, Pb(C2H3O2 )2 , 100 mL (32.5g Pb(C2H3O2 )2 per 100 mL solution)
1.0 M Potassium iodide, KI, 100 mL (16.6 g KI per 100 mL solution)
1.0 M Copper(II) sulfate, CuSO4 , 100 mL (25.0 g CuSO4
. 5H2O per 100 mL solution)
1.0 M Sodium chromate, 100 mL (23.4 g Na2CrO4 ·4H2O per 100 mL solution)
1 Iron nail
1 Zinc strip
1.0 M Acetic acid, HC2H3O2 , 100 mL (mix 6 mL glacial acetic acid per 100 mL solution)
Hot plate
Sodium silicate solution, 100 mL (15 mL of saturated solution diluted to 100 mL).
The saturated solution can be purchased in hobby shops or pharmacies as water glass. If
not available locally, Aldrich Chemical Co., (800) 558-9160, will send 1 liter (Cat. No.
33, 844-3) for $12.60 plus shipping charges.
Safety
Dispose of solutions according to local codes.
Procedure
- Preparation of acidified gel.
- Prepare a hot water bath by half filling a 400-mL beaker and placing it on a hot plate.
- Put 25 mL 1.0 M acetic acid in a test tube. (NOTE: The concentration of acetic acid and the sodium silicate solution are
critical for gelling.)
- Add the specified amount of the first reactant (see following table) to the acetic acid
in the test tube, and mix well. This may be done by stoppering the test tube and gently
turning it upside down several times.
- Add 25mL sodium silicate solution to the acid mixture. Mix well as above, and cover with
Parafilm (with a pinhole) or a stopper.
- The gel will set in minutes if put into very hot water.
- Add the metal or solution listed for specific systems (see following table), stopper,
and display.
- Observe daily.

Demonstration 2: An Inorganic Polymerization
Demonstration
Description
Silicates polymerize in acid solution according to
the following equation:
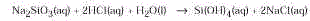
Although the product is represented by the formula Si(OH)
4 , it consists of a complex
mixture of polymeric acids formed by condensation.

The Si(OH)
4 cannot be isolated from the mixture. If the gelatinous mixture is heated, the
reaction is reversible. If the NaCl(aq) is removed by washing, the reaction is not
reversible. On heating, the unwashed silica becomes a solid, and the washed product is a
powder that can absorb a large amount of water.
Materials
- 1 M Hydrochloric acid, HCl, 100 mL (8.4 mL conc. HCl diluted to 100 mL. Be sure to add acid to water.)
- 17% Sodium metasilicate, Na
2SiO3 , 100 mL (water glass, described in demonstration. 17 g Na2SiO3 is mixed with 83 g H2O.)
Beaker, 250-mL
2 Petri dishes
Drying oven or microwave oven
0.05 M Silver nitrate, AgNO3 , 10 drops (0.02 g in 2 mL distilled H2O)
Distilled or deionized water
Procedure
Add 100 mL 1 M HCl to 100 mL 17% Na2SiO3 in a weighed 250-mL
beaker. Decant supernatant liquid.
Find the mass of the gel. Divide the gel into 2 equal parts.
Place half in a weighed Petri dish, and weigh again.
Wash the remaining gel in the beaker with distilled water until no cloudiness appears
when a few drops of AgNO3 is added to the decanted wash water.
Heat both gels in a drying oven at 105 °C for 1.5
hr or in a microwave oven for 15 min.
Reweigh the samples.
Data Analysis
- Describe the differences in the two samples.
- Students can calculate the number of grams of water absorbed
by one gram of gel for each sample.
- Explain the differences in structure that can account for
the results in Question 2.
- Which of the samples would be most likely to be packed with
cameras and electronic equipment for use as a drying agent?
Demonstration 3: Supersaturated Solution
Description
The action of heat and pressure on water beneath the surface of the earth sometimes
creates supersaturated solutions. This demonstration shows how such solutions could
produce crystals.
Materials
- Sodium acetate trihydrate, NaC
2H3O2 . 3H2O, 160 g
Distilled water, 30-mL
Tap water for water bath
Erlenmeyer flask, 500-mL
Beaker, 1-L or greater for water bath
ParafilmÔ or 100-mL beaker to cover the Erlenmeyer flask
Graduated cylinder, 50- or 100-mL
Glass stirring rod
Wash bottle with distilled water
Balance, triple beam
Hot plate, laboratory burner or alcohol burner
Ringstand set up, if using burner
Heat-resistant gloves or tongs
Goggles and apron
Procedure
Weigh 160 g of sodium acetate trihydrate in a 500-mL Erlenmeyer flask.
Add 30 mL distilled water to the flask.
Heat the mixture in a hot water bath stirring occasionally
until all of the solid is dissolved. (This may take 15 min or so.)
Remove all crystals from the sides of the flask by rinsing
them down with small squirts of water from the wash bottle.
Cover the flask with Parafilm or the inverted 100-mL beaker.
Allow the solution to cool to room temperature undisturbed,
or to speed up the cooling process, run cold water over the sides of the flask making sure
no tap water gets into the flask and contaminates the solution.
While holding a single crystal of sodium acetate over the
open mouth of the flask, snap your fingers, and allow the crystal to drop into the flask.
The single crystal should start crystallization.
After crystallization is complete turn the flask upside
down, and nothing should fall out.
The sides of the flask should be warm since this is an
exothermic process.
The solution may be used over and over again by reheating it
to redissolve the sodium acetate.
Remarks
The addition of too much water will result in
leftover liquid after recrystallization. Variations of this demonstration include
placing a single crystal of sodium acetate in a shallow container and pouring the solution
described above on the crystal to produce a "stalagmite" of sodium acetate. A
buret may also be used to add the solution to the crystal.
Demonstration 4: Precipitation and Redissolution of
Calcium Carbonate
Description
CaCO3 is the major chemical component of many minerals and natural
products. These minerals include marble, calcite, aragonite, coral, sea shells,
stalactites, stalagmites. Precipitation of CaCO3
forms these substances. Dissolution of CaCO3 allows the raw
materials to be transported.
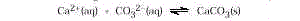
This demonstration illustrates how sensitive the direction of this equilibrium
expression is to reaction conditions (pH, carbonate concentration, temperature).
Materials
2 (limewater)
[Boil 1 L distilled water, cover, and
allow to cool overnight. This removes CO2 from the water. Add 1.8 g
Ca(OH)2
, mix well, and allow to
settle. Filter if cloudy at the time of use.]
Carbon dioxide, CO2
(either dry ice (solid CO2 ) or a cylinder of compressed CO2 )
4 Straws (or 25-cm lengths of 6- to 8-mm glass tubing, fire
polished)
Gloves or towels to handle dry ice
Hot plate with magnetic stirrer and stirring bar
2 Beakers, 500-mL
Safety
The Ca(OH)2
solution is basic with a pH = 12.4. It can irritate
eyes and skin. In case of ingestion, give copious drinks of water to dilute the
limewater in the stomach, and seek medical advice.
Procedure
- Place 150-250 mL of clear, saturated Ca(OH)
2 in a 500-mL beaker.
Have a volunteer blow gently through a straw or glass tube into the solution.
Caution: because Ca(OH)2 is a strong base, warn the volunteer to not get any of
the liquid into the mouth (see Safety above). A white precipitate of CaCO3
will form.
Place a second sample of Ca(OH)2 in a second beaker.
Bubble pure CO2 through the solution by dropping in a piece of dry ice or by using the compressed
gas. Initially, a white precipitate of CaCO3
will form. However, the precipitate will slowly
dissolve eventually giving a clear solution.
Have your volunteer return to try to redissolve the first
sample of CaCO3 by blowing again into the sample. No amount of blowing will
redissolve the precipitate.
Put the solution from Step 2 on a hot plate with stirring.
The carbon dioxide gas is less soluble in hot water and is removed. Near the boiling
temperature, the solid begins to reform. When the solution cools, the appearance of
suspended solid will be quite pronounced. (Adding pure CO2 to this final solution will not
redissolve the CaCO3 .)
Explanations
Step 1. Although H2CO3 is not stable, it is
useful to view CO2 dissolving inwater to form carbonic acid.
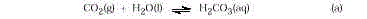
The acid is immediately neutralized by hydroxide forming carbonate ion that in turn
reacts with the calcium ion forming insoluble CaCO
3
.
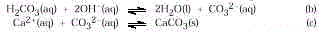
Step 2. When pure CO
2 is being added, its concentration increases until all the hydroxide
originally in the solution is consumed. Thus, any excess dissolved CO2 is unneutralized carbonic
acid. Some hydrogen ions dissociate and dissolve the precipitate converting the carbonate
to bicarbonate. (CaHCO3 is soluble.)
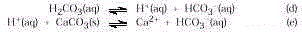
Step 3. Exhaled breath is only 4% CO
2 . By contrast, the gas obtained from dry ice
is 100% CO 2. Blowing into the solution never provides
a concentration of carbonic acid high enough to exceed the available hydroxide. Think of
all the reactions as reversible. Thus, in each, the reactants and products are in a state
of balance. When the pressure of CO 2 (g) in reaction
(a) cannot get very high, reaction (b) cannot be forced to consume all the OH – . Thus the effect of the last two reactions cannot be
seen. That is, the equilibrium in reaction (d) and (e) lies far to the left.
Step 4. Heating the solution decreases the solubility of CO
2 in water. Thus reaction (a) is
forced to the left. This in turn forces reactions (d) then (e) to the left and CaCO 3 reforms.
Demonstration 5: Pot-O-Gold
Purpose
This demonstration should precede a discussion on
solubility. Solubility and precipitation are important in understanding the occurrence of
minerals. This demonstration catches the attention of students who watch the platelets
swirling and catching the light.
Materials
Nonconsumables
- Beaker, 1-L
- Florence flask, 500-mL, with stopper
- Burner
- Funnel
- Consumables
- 0.20 M Lead nitrate, Pb(NO 3 ) 2 , 50mL (3.3g Pb(NO 3 ) 2 per 50mL solution)
- 0.20 M Sodium iodide, NaI, 50mL (1.5g NaI per 50mL solution)
- Filter paper
Safety
Both lead and iodide ions are listed as hazards.
Students should not handle these chemicals. Normal precautions should be followed. Excess
solid lead iodide should be stored in a lead-waste container. The filtrate is used as part
of the second solution.
Procedure
- Mix the lead nitrate and sodium iodide solutions. A yellow
precipitate of lead iodide will form.
- Filter the supernatant liquid into 1-L beaker and add enough
distilled water for a total volume of 300 mL.
- Heat solution containing the filtrate to boiling.
- Add enough solid lead iodide to make a saturated solution at
100°C.
- Pour the saturated solution, while hot, into a 500-mL
Florence flask.
- Let the solution cool slowly to room temperature. The lead
iodide will precipitate out as shiny platelets that look like gold.
- Stopper the flask securely. The mixture can be kept in a
stoppered flask for several years.

![]()

![]()
![]()
![]()
![]()