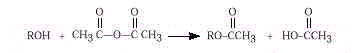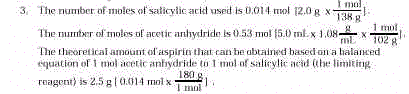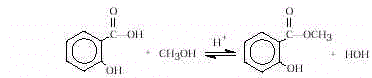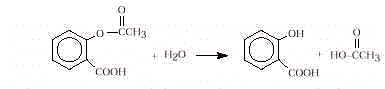LABORATORY ACTIVITY: STUDENT VERSION
Activity 1: Synthesis of Aspirin
Introduction
In this laboratory activity you will synthesize aspirin, a
derivative of salicylic acid. Salicylic acid and its derivatives are antipyretics. Such
compounds lower the body temperature of a person with a fever. They have little effect if
the body temperature is normal. Salicylates are also mild analgesics that relieve pain
associated with headache, neuralgia, and rheumatism. Salicylic acid itself is not used for
these purposes because it has an irritating effect on the stomach.
The most common salicylate used in medicine today is
aspirin. In this activity, aspirin is prepared from salicylic acid and acetic anhydride.
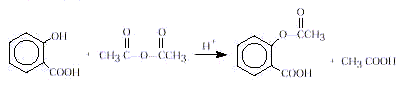
When ingested, aspirin (acetylsalicylic acid) passes
through the stomach largely unchanged. It is hydrolyzed in the intestinal tract liberating
the active ingredient, salicylic acid.
In this laboratory synthesis, you will heat a mixture of
salicylic acid and acetic anhydride with a trace of sulfuric acid as catalyst. Because
aspirin is not very soluble in water, it can be isolated by addition of cold water to the
reaction mixture followed by a gravity filtration.
Purpose
To synthesize aspirin from salicylic acid, test for the
purity of the product obtained, and determine the number of aspirin tablets that can be
obtained from your preparation.
Safety
- Wear protective goggles throughout the laboratory activity.
- Acetic anhydride is irritating in the liquid and vapor
state. Avoid contact with skin and eyes. Rinse with water any body area that comes in
contact with acetic anhydride.
- Concentrated sulfuric acid is a powerful dehydrating agent;
handle it carefully to avoid contact with skin and clothing.
- Methanol is toxic; breathing its vapors and skin contact
with methanol should be avoided.
Procedure
- Set up a ring stand with a ring, wire gauze, and burner. The
top of the burner should be about 12 cm below the ring and wire gauze. Place on the wire
gauze a 400-mL beaker half-filled with water. Heat the water to boiling. This is your
water bath. As you wait for the water to boil, go to the next step.
- Weigh 2.0g salicylic acid on weighing paper, and transfer
the solid to a 125-mL Erlenmeyer flask.
- Add 5mL acetic anhydride from a 50-mL buret.
- Add 5 drops concentrated sulfuric acid. (CAUTION: Sulfuric
acid is corrosive.) Stir the mixture.
- Heat the flask in the boiling water bath for 10 min.
- Remove the flask from the water bath, and carefully add 2mL
water from a 10-mL graduated cylinder. Swirl to mix the contents. Allow the flask to stand
for 5 min.
- Add 40mL water from a graduated cylinder, and stir the
solution until crystals begin to form.
- Cool the flask in an ice-water mixture in a 400-mL beaker
for 10 min to complete the crystallization.
- Collect the product by gravity filtration using a 250-mL
beaker to collect the filtrate. Wash the product twice with 5mL cold water.
- Let the aspirin dry until the next laboratory period.
- Transfer the dried aspirin to a weighed piece of filter
paper and weigh. Calculate the number of aspirin tablets you have prepared:
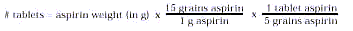
- Calculate the percent yield of your aspirin from the formula
below. The quantity in the denominator (2.5 g) represents the theoretical yield of aspirin
based on the moles of salicylic acid and acetic anhydride used in the synthesis.
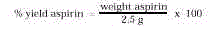
- Determine the qualitative purity of your aspirin in the
following manner. Place 1mL methanol in each of three separate test-tubes. Add a few
crystals of the following: Test-tube 1, salicylic acid; Test-tube 2, your prepared
aspirin; and Test-tube 3, commercial aspirin, crushed. Add 1 drop of 1% iron(III) chloride
solution to each test-tube. Observe the color in each tube. What can you conclude?
- Extension: Determine the melting point of the aspirin as
directed by your instructor.
- Place the aspirin in a dry test-tube, label with your
name(s), and give it to your teacher.
- Thoroughly wash your hands before leaving the laboratory.
Data Analysis and Concept Development
- Can the purity of the prepared aspirin be determined by the
color test with the iron(III) chloride?
- Can the purity of the prepared aspirin be determined by the
melting point?
- In Step 12, the quantity 2.5g represents the theoretical
yield of aspirin. Show the calculation leading to a theoretical yield of 2.5g of aspirin.
[You must first determine the limiting reactant from the following data: Molar mass of
salicylic acid = 138 g; molar mass of acetic anhydride = 102 g; density of acetic
anhydride 1.08 g/mL.]
Implications and Applications
- What steps do aspirin manufacturers have to take to make
sure that theirproduct is fit for human ingestion?
- What other types of analgesics are on the market? How does
their action compare with aspirin and why are these alternative drugs necessary?
LABORATORY ACTIVITY: TEACHER NOTES
Activity 1: Synthesis of Aspirin
Major Chemical Concept
The synthesis of aspirin is known in organic chemistry as
an esterification reaction. This is a substitution reaction in which an alcohol (the
–OH group in salicylic acid) reacts with acetic anhydride to form an ester, aspirin.
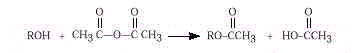
Level
Honor chemistry students
Expected Student Background
None expected. The techniques illustrated here (synthesis,
melting point determination) are not likely to have been previously encountered.
Time
The activity will take one 50-min period for the synthesis,
an overnight drying step, and a second period for calculations, color tests, and melting
point determination.
Safety
Read the Safety Considerations in the Student Version. The
acetic anhydride,methanol and concentrated sulfuric acid should be handled with care.
Dispensing the anhydride from a buret minimizes spills and possible body contact.
Concentrated sulfuric acid should be dispensed from a small dropping bottle. The aspirin
produced in this activity is not pure. It is often contaminated with salicylic acid,
acetic acid, and/or sulfuric acid. Students should not taste their aspirin.
Materials (For 24 students working in pairs)
Nonconsumables
- 12 Melting point devices (see Possible Extensions )
- Mineral oil, 1 L
- 12 Thermometers (50-150 °C)
- Capillary melting tubes
- 12 Funnels and supports
- 4 Burets, 50-mL
- 12 Erlenmeyer flasks, 250-mL
- 24 Beakers, 400-mL
- 12 Beakers, 250-mL
- 12 Graduated cylinders, 10-mL
- 12 Graduated cylinders, 50-mL
- 12 Flat-tipped spatulas or "rubber policemen"
- 12 Ring stands, rings and wire gauze
- 12 Stirring rods
Consumables
- Salicylic acid, 24 g
- Acetic anhydride, 60mL
- Concentrated sulfuric acid, 5 mL (in dropping bottles)
- Crushed ice
- Methanol, 50 mL
- Commercial aspirin sample
- 1% Iron(III) chloride, FeCl 3 , 100 mL (1g FeCl 3 per 100mL solution)
Advance Preparation
Fill the 50-mL buret with acetic anhydride. Prepare 1%
iron(III) chloride solution in dropping bottles. Set out container of salicylic acid. Use
of vacuum filtration, if available, will facilitate the filtration. Purchase commercial
aspirin tablets, or obtain samples from the school health office. Set up mineral oil baths
for melting point determination as shown in Possible Extensions.
Pre-Laboratory Discussion
- Briefly explain the reaction type (substitution,
esterification) and synthetic goal.
- Caution students about reagent hazards.
- Review the use of a buret for delivering a given volume of
liquid.
- Review technique of stirring versus swirling.
- Review theoretical yield calculation.
- Explain the concept in the iron(III) chloride test. Many
phenols form colored coordination compounds with iron(III) ion, in which six molecules of
a monohydric phenol are combined with one atom of iron in the form of a complex anion.
Salicylic acid contains a phenolic –OH group and gives a positive test (turns purple)
with iron(III) chloride. Complete reaction of salicylic acid with acetic anhydride
substitutes an ester for the phenolic group. Therefore, aspirin uncontaminated with the
salicylic acid starting material will give a negative test with Fe 3+ . What can students conclude if their
aspirin preparation turns purple? [The preparation still has salicylic acid starting
material present.]
- Explain how to obtain a melting point range (temperature at
which solid first melts to the temperature at which solid is melted).
Teacher-Student Interaction
Walk around room during laboratory exercise emphasizing
proper technique. Talk with students to ascertain if they understand activity. Help with
yield calculations and melting point determination.
Anticipated Student Results
Typically students obtain a 60-70% yield. The product is
often contaminated with salicylic acid. The result of the iron(III) chloride test is:
salicylic acid (dark purple), prepared aspirin (dark purple), and commercial aspirin
(light salmon). The melting point range is broad, i.e., there is a large temperature range
between first melting and complete melting of the sample. Typical values are between
128-137 °C.
Recrystallization of the aspirin would remove unreacted salicylic acid and narrow the
melting point range.
Answers to Data Analysis and Concept Development
- Yes. The prepared aspirin should not give a positive color
test with the iron(III) chloride because salicylic acid, which gives the positive test, is
absent.
- Yes. The sharpness of the melting point is one of the best
methods of determining purity of an organic solid. A broad melting point indicates the
presence of impurities. In this case, the impurity is probably a trace of unreacted
salicylic acid.
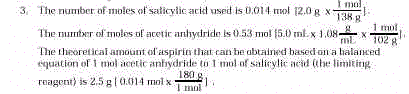
Answers to Implications and Applications
- Purification by recrystallization and testing for impurities
by melting point determination and chromatography are necessary. Strict regulatory codes
apply.
- Other analgesics may be anti-inflammatory or antipyretic,
but only aspirin is a "triple action" drug (fulfills all three functions).
Aspirin is sometimes irritating to the digestive tract lining, so alternatives must be
available for individuals affected by aspirin in this way.
Post-Laboratory Discussion
Review the experimental techniques, the experimental
results and their significance.
Possible Extensions
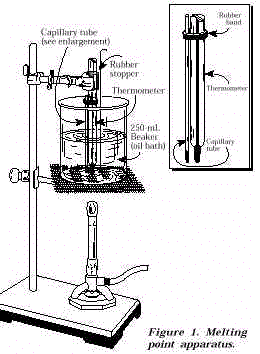
- Determine the purity of your aspirin by obtaining its
melting point. Use the set-up shown in Figure 1. Crush about 50-100 mg of dry aspirin with
a spatula against the walls of a 50-mL beaker. Thrust the open end of a melting point
capillary tube into the powdered aspirin several times. Work the plug of solid material
down to the tube’s sealed end by dropping it 3-4 times into the open end of the 50-mL
beaker used for crushing. The capillary tube should contain the amount of packed solid as
shown in Figure 1. Attach the capillary tube to the thermometer as shown in Figure 1. The
rubber band can be a small section of rubber tubing of suitable diameter. Place the
thermometer and tube in the 250-mL beaker oil bath. Heat the oil bath so that the
temperature rises about 1-2 °C/min. Record as the melting point range the temperature at which the sample
begins to melt and the temperature at which the sample is completely melted. The melting
point of aspirin is about 135 °C. Discard the used melting point tubes in a waste glass container.
- Synthesize methyl salicylate (oil of wintergreen) in a medium-sized test-tube from 0.5g
salicylic acid and 1mL methanol. Stir the mixture, and add 3 drops of concentrated
sulfuric acid. Heat the mixture in a water bath for 5 min. Remove the test-tube, cool it
in a test-tube rack, and then add the contents to a 100-mL beaker containing 4-5 ice
cubes. Waft the odor toward thenose. What commercial products contain oil of wintergreen?
[Wintergreen Life-Savers, Ben-Gay, etc.]
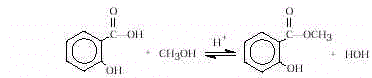
- Use a Spectronic 20 to determine the amount of salicylic acid in the
laboratory-synthesized aspirin and in commercial aspirin. To prepare an aspirin solution,
dissolve 0.05g aspirin in 50mL water. Prepare an iron(III) nitrate solution by dissolving
2g iron(III) nitrate nonahydrate [Fe(NO 3 ) 3 . 9 H 2 O] in 50mL water
and adding 0.5mL concentrated nitric acid. To 1mL aspirin solution add 5mL iron(III)
nitrate solution. Shake well for 5min (a violet color should appear if salicylic acid is
present). Measure the absorbance at 535–540 nm by placing the violet solution in the
cell of a Spectronic 20 instrumentafter standardizing with distilled H 2 O. NOTE: Old Spectronic 20’s may still have their wavelength
scales calibrated in mm (millimicrons) but the
accepted term for this unit is nanometers(nm). The absorbance is proportional to the
concentration of salicylic acid in the sample. Compare your laboratory aspirin with
several brands of commercial aspirin.[The laboratory synthesized aspirin should have an
absorbance >0.5 at 540 nm. The commercial aspirin has an absorbance of <0.2 at 540
nm. The commercial aspirin solution does not change to a violet color when mixed with the
iron(III) nitrate solution.]
Assessing Laboratory Learning
- What are the most likely impurities present in the sample of aspirin you prepared?
[Unreacted salicylic acid, traces of acetic acid.]
- If aspirin sits for long periods of time, the odor of vinegar can be noticed in the
container. A hydrolysis reaction occurs very slowly. Explain, using a chemical
equation,thesourceof thevinegar,andthe reasonforthehydrolysis. [Slow reaction with
moisture in air can produce small amounts of vinegar.]
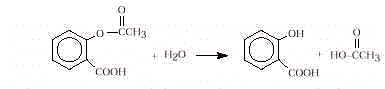
- A white powder was tested by a police chemist with iron(III) chloride solution. A purple
color is seen. What conclusion was drawn by the police chemist?[The white powder contained
a phenolic compound, possibly salicylic acid.]
- Aspirin tablets are sold as containing 5grains of aspirin. If 1 grain equals 65mg, how
many milligrams of aspirin does each tablet contain? [325mg]


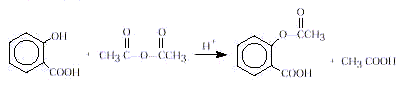
![]()
![]()