Demonstration
2: Colloids, The Tyndall Effect and More
Introduction
The
Tyndall effect is usually given as a definitive test to distinguish between
a true solution and a colloid. The Tyndall effect involves the scattering
of a beam
of light as the light passes through a medium having particles of colloidal
size. Since particles such as molecules of sugar or sodium ions or chloride
ions in
solution are too small to scatter light, a beam of light passing through
such a solution is not scattered. However, the protein molecules in milk
are of colloidal
size and consequently a drop of milk mixed into water will cause a light
beam traversing the solution to be scattered. To demonstrate the difference,
the two
systems described above are usually employed. The milk in water changes
color as more milk is added (bluish to yellow to red). However, a single
system
where the particles go from “solution size” to “colloidal size” provides
a more dramatic demonstration and as the articles continue to grow additional
optical
effects may be demonstrated. One such system is the production of sulfur
by the reaction between sodium thiosulfate and sulfuric acid.
Na 2 S 2 O 3 (aq) + H 2 SO
4
(aq) ® S(s) + SO 2 (g) + H 2 O(l) + Na 2
SO 4 (aq)
Here,
the particles of sulfur grow from solution size to colloidal size and finally
begin to precipitate. As this phenomenon occurs, it is possible to demonstrate
the Tyndall effect and to examine some of the characteristics of the scattered
and transmitted light. These characteristics may then be related to other
phenomena, such as red sunsets.
Safety
Some
students are very allergic to sulfur dioxide (SO 2 ), which
is generated during this demonstration. You should offer a warning and
excuse students if
they have a known sensitivity to sulfur dioxide (or to sulfites in food,
which may signal such a sensitivity).
Option A
Materials
Square or rectangular battery jar or a small fish tank
Stirring rod (long glass rod to stir contents of the battery
jar)
Parallel beam light source or a flashlight
Piece of frosted glass or a sheet of white paper stapled to a
frame
Ring stand and clamp to hold the glass or paper in a vertical
position
Preweighed sample of Na 2 S 2 O 2
or Na 2 S 2 O 3 ·5H 2
O to make a 0.01 m solution when dissolved in the water in the battery
jar (1.6 g Na 2
S 2 O 3 per L water or 2.5 g Na 2
S 2 O 3 ·5H 2 O per L water) 10
mL Concentrated sulfuric acid, H 2 SO 4 per L of
water in the battery jar
Optional:
One or more pieces (sheets) Polaroid material
Cardboard silhouette of a flying duck (about the size of half
the diameter of the parallel beam) and hung like a mobile from a fine piece
of string
Recording of “Canadian Sunset” and appropriate player.
Directions
The demonstration should be conducted in a well ventilated area because
of the production of SO 2 (g) whose odor can be mildly detected
while the demonstration is in progress.
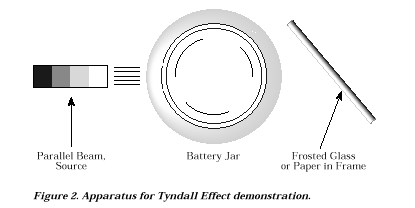
Set up the light source, battery jar, and frosted glass as illustrated
in Figure 2. The battery jar should be filled with sufficient depth of
water so that the entire diameter of the light beam passes through the
liquid. (The amount of water should be predetermined in order to have a
preweighed sample of sodium thiosulfate and a premeasured volume of concentrated
sulfuric acid prepared.) Also, the water should be placed into the battery
jar a few minutes prior to beginning the demonstration to permit air bubbles
to escape.
Turn on the light source and darken the room. If you will be using the
Polaroid sheets, pass these out to students seated directly in front of
the demonstration. Ask students whether they can see the beam traversing
thewater. If using the Polaroid sheets, ask whether rotating them has any
effect. [They should not be able to see the beam but the white round
disk of the transmitted beam hitting the frosted glass should be visible.
Nothing different should be seen using the Polaroid sheet.] Point out that
the transmitted beam striking the frosted glass is colorless (white). Add
the preweighed sample of sodium thiosulfate to the water and stir until
it is dissolved. Wait for any bubbles to leave the solution. Repeat
the above question(s). [The reply should be the same.] Carefully and with
stirring, add the premeasured concentrated sulfuric acid to the sodium
thiosulfate solution. Wait about 15 seconds for bubbles to clear and repeat
the above question(s). [The reply should be the same.] Ask if there is
any evidence of a chemical reaction? [There should be no such evidence.]
Ask students to tell you when they notice any change. [Using the concentrations
recommended and a solution temperature of about 20 °C, it should take
about two minutes for the Tyndall beam to start to appear and another two
minutes for the particles to become large enough and concentrated enough
for the percent of transmitted light to go to zero. The reaction rate appears
to be first order in thiosulfate concentration so if you want to slow things
down, decrease the concentration of sodium thiosulfate.] As soon
as the Tyndall beam becomes visible ask students to compare the color of
the scattered light to that of the transmitted light striking the screen.
Also ask students using the Polaroid sheets whether rotating them has any
effect on the intensity of the scattered light. [The scattered beam is
bluish, whereas the transmitted light starts to turn yellow. As the Tyndall
beam becomes more visible the scattered beam loses a little of its bluish
color as the green and yellow portion of the spectrum is scattered and
the transmitted
light striking the screen goes from yellow to red. This observation
occurs because the wavelengths of the light scattered are directly proportional
to the size of the particle scattering the light. Also, the scattered light
is polarized.] If you have the silhouette of a “flying duck” you can add
a dramatic closing touch to the demonstration. Start the recording of “Canadian
Sunset” and hang the silhouette over the frosted glass so that it intercepts
the disk of the reddening transmitted beam before the beam strikes the
glass.
T O P V I E W
Dispose of the battery jar solution as soon as the demonstration is ended
to minimize the amount of SO 2 entering the atmosphere. After summarizing
or students that the Tyndall beam was not noted until the particle size
had become that of a colloid and that no beam had been visible when there
was a
true solution, you might want to extend the discussion and demonstration
to other areas. If the day happens to be sunny with a relatively clear
sky you
might ask students to use the Polaroid sheets to observe the light coming
from the blue sky at right angles to the direction of the sun. (CAUTION:
Make sure students do not look toward the sun since this is very dangerous.)
[The scattered blue light is polarized.]
Questions
You may follow the demonstration with the following questions:
1. Why does the sun appear exceptionally red when it sets behind a city?
[The blue end of the visible
spectrum is scattered by the dust and aerosols in the air over the city
and the red end of the spectrum is
transmitted.]
2. Why are fog lights usually yellow and not white? [The yellow light being
of relatively long wavelength is
transmitted through the colloidal size fog particles thereby permitting
the driver to see, whereas the blue
component of white light is scattered back to the driver thereby obscuring
vision.]
3. Why not use red fog lights? [Red light is usually a signal of danger
and approaching motorists may interpret
it as such. Also, the human eye is much more sensitive to yellow light
than red light.]
4. Why do things look clearer through rose (pink) colored glasses? [The
pink glass filters out blue light that is
scattered by aerosols in the environment. Since this blue light does not
reach our eyes, the clarity of objects
is increased.]
Option B
Materials
Overhead projector
Medicine dropper
Beaker of water
Milk, 10 mL
Directions
A simpler and quicker demonstration involves a beaker of water on the stage
of an overhead projector and a small amount of milk in a medicine dropper.
As the milk is slowly added to the water, the color of the water becomes
bluish, while the circle of light thrown up onto the screen gradually becomes
yellow then orange and then red. The questions suggested above (Option
A) are also appropriate for this option.