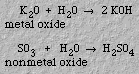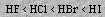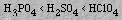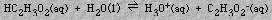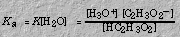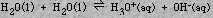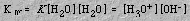Return to Table of Contents
Within Chemistry
1. Periodicity
Metal oxides usually form basic solutions in water; aqueous solutions of nonmetal oxides are acidic.2. Solutions/solubility
The acidity of the hydrogen halides increases in the sequence
The basicity of Period 3 hydroxides decreases in the sequence
The acidity of Period 3 oxyacids increases in the sequence
Most acids tend to be water soluble. NaOH and KOH are quite soluble in water. Ca(OH)2 is moderately water soluble. Acidic solutions dissolve some metals (Fe, Zn, and Al), releasing hydrogen gas. Zinc and aluminum will dissolve in base. Drano(TM) consists of solid NaOH and Al turnings. When water is added to the mixture, hydrogen gas is evolved.3. Equilibrium; Ionization Constant
The dissociation of a strong acid or base is complete. The ionization constant expression is a measure of the equilibrium for the ionization of a weak acid or base. For example, the equilibrium established when the weak acid, acetic acid, ionizes,
is given by the ionization constant expression:
Water has the important characteristic of being able to act as either an acid or base. This tendency allows two water molecules to react accordingly
The equilibrium constant expression for the process is
Kw, the ion product constant for water, has a value of 1.0 x 10-14 at 25 oC and 2.4 x 10-14 at 37 oC (body temperature). This is significant because it means that although at 25 oC a neutral solution has a pH = 7, at 37 oC, the pH of a neutral solution is less than 7 (6.81).
(Page 31)
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|