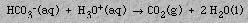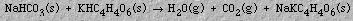Magnesium, Mg, is the lightest structural metal in common use. It is
obtained from sea water. Lime, CaO, is added to sea water to precipitate
magnesium hydroxide, Mg(OH)2. Magnesium hydroxide is then filtered and
neutralized with hydrochloric acid. After evaporation of the water, the
resulting MgCl2 is melted and electrolyzed to give magnesium metal.
After recovery from coal and petroleum or even sulfur mines, sulfur is
converted to sulfuric acid in four steps: (1) the sulfur is burned in air to
sulfur dioxide, SO2; (2) sulfur dioxide is passed over a hot platinum surface
where it is converted to sulfur trioxide, SO3; (3) sulfur trioxide is passed
into sulfuric acid where pyrosulfuric acid, H2S2O7, is formed, and (4) the
latter is diluted with water to give sulfuric acid, H2SO4. Sulfuric acid is the
number one chemical substance in terms of total mass produced by U.S.
industry.
Sodium hydroxide is prepared by electrolysis of a concentrated aqueous sodium chloride solution.
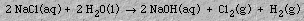
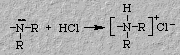 Lidocaine and novocaine are bases that are used by the medical
profession as anesthetics. Because of their limited solubility in water (the
typical injection solvent), the basic amino group in these molecules is
converted to their hydrochloride salt form which is water-soluble. Furthermore, as bases these drugs would cause localized changes in pH at the injection site. Thus, the use of salt solutions minimizes this localized irritation.
Lidocaine and novocaine are bases that are used by the medical
profession as anesthetics. Because of their limited solubility in water (the
typical injection solvent), the basic amino group in these molecules is
converted to their hydrochloride salt form which is water-soluble. Furthermore, as bases these drugs would cause localized changes in pH at the injection site. Thus, the use of salt solutions minimizes this localized irritation.
Antacids are basic compounds that decrease the amount of hydrochloric acid in the stomach. Examples are milk of magnesia (magnesium hydroxide), Mg(OH)2, and Rolaids(TM) (sodium dihydroxy aluminum carbonate, NaAl(OH)2CO3). Sodium bicarbonate (baking soda), NaHCO3, is the antacid in Alka Seltzer(TM). When Alka Seltzer(TM) is placed in water, bicarbonate ions react with hydronium ions from the acid producing the familiar fizz and belch:
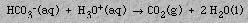
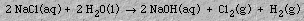

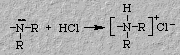 Lidocaine and novocaine are bases that are used by the medical
profession as anesthetics. Because of their limited solubility in water (the
typical injection solvent), the basic amino group in these molecules is
converted to their hydrochloride salt form which is water-soluble. Furthermore, as bases these drugs would cause localized changes in pH at the injection site. Thus, the use of salt solutions minimizes this localized irritation.
Lidocaine and novocaine are bases that are used by the medical
profession as anesthetics. Because of their limited solubility in water (the
typical injection solvent), the basic amino group in these molecules is
converted to their hydrochloride salt form which is water-soluble. Furthermore, as bases these drugs would cause localized changes in pH at the injection site. Thus, the use of salt solutions minimizes this localized irritation.