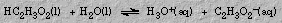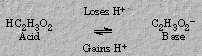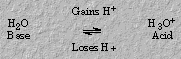The Brønsted-Lowry definition of an acid is a species that donates protons, and a base is a species that accepts protons. These definitions have a valuable application in interpreting experimental facts. The dissociation of an acid (or a base) is an equilibrium reaction. For example, in the dissociation of acetic acid,
acetic acid donates a proton to water. Acetic acid is a Brønsted acid. Water, which accepts the proton, is a base.
In the reverse reaction (which is proceeding at the same rate), hydronium ion donates a proton to acetate ion. Hydronium ion is an acid. Acetate ion is a base because it accepts a proton.
Acetic acid by losing a proton forms a base, acetate ion. Acetate ion, by accepting a proton, can form an acid--acetic acid. This relationship can be represented as follows:
This acid-base pair, formed from each other by the loss or gain of a proton represents a conjugate acid-base pair. Conjugate means joined in pairs, or coupled.
Similarly, water (a base) accepts a proton to form the acid hydronium ion, which in turn, forms water by loss of a proton.
Hydronium ion and water are a second conjugate acid-base pair in the acetic acid dissociation.
When a salt dissolves in water, it completely dissociates to produce cations and anions. Either one or both of these ions may then react with water. This reaction is called hydrolysis. Hydrolysis of a salt can affect the pH of the solution. The resulting pH depends on the nature of the salt dissolved in water. (See Figure 21.)

(Page 36)
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|