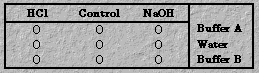
Set up a 3 x 3 matrix of test tubes.
Add Buffer Solution A (acetic acid buffer) to three test tubes. Place
distilled water into the second tube of each set. Then add Buffer solution B
(ammonia buffer) to the last three test tubes. Add a drop or two of universal
indicator solution to each tube; stir with a stirring rod until a brightly
colored solution results. To the first of each of the three test tube sets, add
a dropperful of 6 M HCl; to the right hand member of each of the three test
tube sets, add a dropperful of 6 M NaOH. Stir the solutions. Have students
record observed changes. To illustrate the capacity of the buffer, slowly add 6 M NaOH to the third test tube in the acid buffer set (Set A) and 6 M HCl to the third test tube in the basic buffer set (Set B) until pronounced color changes are noted.
The Brønsted-Lowry concept of an acid and a base was limited to
electron pair sharing to the proton (H+). A new concept of acids and
bases was proposed in the early 1920s by G. N. Lewis, a professor of chemistry at the University of California at Berkeley. In the Lewis proposal, an acid is an electron-pair acceptor ("take two from you"), a base is an electron-pair donor ("have pair, will share"), and an acid-base reaction involves a base sharing an electron pair with an acid.
Lewis extended the Brønsted-Lowry definition of an acid and a base.
Lewis acids and bases are not dependent upon the proton (H+) or the
hydroxide ion. In fact, the Lewis concept revolutionized the theory and
practice of acid catalysis in organic chemistry (see last equation in Figure
21-formation of a carbocation).
In a Lewis acid-base reaction a coordinate covalent bond is formed between an acid and base. Examples are in the equations shown in Figure 21:




