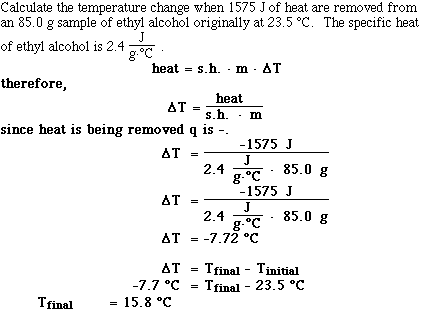
In our studies thus far we have written simple equations,
predicted the products of simple reactions, balanced equations
and performed quantitative calculations using balanced chemical
equations. There is one important characteristic of a chemical
reaction which we have not yet included in a chemical reaction.
The heat which is involved in the chemical reaction.
We have seen several reactions which we could classify as
violent. Recall the reaction between aluminum and bromine.
Although the reaction was slow to start, once it began it
proceeded rapidly. We saw bromine vapor bellowing out of the
beaker and pieces of aluminum glowed red hot as they danced about
on the surface of the liquid bromine. The reaction of potassium
in water was another violent reaction.
In class we looked at two new reactions to set the stage for
this discussion.
The first reaction is between aluminum and iron (III) oxide
(Fe2O3). The reaction will be initiated by
adding a few drops of concentrated sulfuric acid to a mixture of
sucrose and potassium chlorate. This 'side' reaction will produce
enough heat to start the reaction between aluminum and iron (III)
oxide.
The reaction is;
Al(s) + Fe2O3(s)
---> Al2O3(s) + Fe(l)
The reaction produces so much heat the product iron changes
phase from solid to liquid. The heat produced is enough to change
the temperature from 25 C to at least 1537 C (the melting point
of iron).
The second reaction is between barium hydroxide and ammonium
chloride. Both of these reactants are white crystalline solids,
as shown here. The two white solids are mixed together in a
beaker which has been placed on a piece of wood (2 x 4) upon
which several drops of water have been placed. Before the
reaction begins the two solids are at room temperature as is the
beaker, 2 x 4 and the water. Show how the beaker does not adhere
to the wood before mixing the reactants.
The first observation is the two solids form a slurry, as a
liquid is formed. This is very interesting, but not surprising
when you are told water is a product of the reaction. The second
observation occurs when the beaker is picked up. The block
adheres to the bottom of the beaker. In fact the beaker and the
block of wood are frozen together. The temperature has fallen
when the two reactants are mixed to the extent the water between
the bottom of the beaker and the block of wood has frozen.
The reaction is;
Ba(OH)2.8H2O(s)
+ 2NH4SCN(s) ----> Ba(SCN)2(aq)
+ 2NH3(g) + 10H2O(l)
As the two white solids were mixed afew students in the front
of the class observed how the temperature of the reaction
changed. The temperature feel is got cooler. We also notice the
change which occurred in the flask. The two solids changed to a
liquid. If we could smell the reaction vessel it would smell of
ammonia.
So what have we discovered in these two examples? These two
reactions were chosen because the particular change I wanted you
to note was very evident. In the case of the reaction between
aluminum and iron(III) oxide the reaction produced heat, in the
form of a flame. Heat was released when the reaction began. In
the case of the barium hydroxide and ammonium thiocyanate instead
of getting hotter the reaction mixture became cooler. In both of
these reactions energy was being transferred in the form of heat.
The study of the energy changes when a reaction occurs is called
thermochemistry. Thermochemistry is part of thermodynamics,the
study of heat, energy, and work and and their transformations.
When discussing heat transfer it is a convenience to identify
where the heat flows from and where it goes to. We will use the
terms system and surroundings to help focus on how heat flows.
System is that portion of the universe we single out for study
and which is bounded by some boundary as defined by a container
or by the sample itself. The surrounding is everything else in
the universe. If in a chemical change or reaction, energy is
released and the temperature of the system will get warmer than
the surroundings, than heat will flow from the system to the
surroundings. This was the case for the reaction between aluminum
and iron(III) oxide.
In the second example the reaction occurred in the beaker.
That was the system. Had we touched the reaction container (the
system) is would have felt cool. Heat would be removed from our
hand, which is at a higher temperature than the system. Heat must
flow from the surrounding into the system.
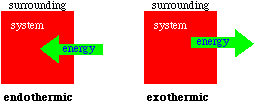
A chemical reaction which releases heat is called an
exothermic reaction. Heat flows from the system to the
surroundings. An endothermic reaction absorbs heat from the
surroundings. To begin our study of thermodynamics (and we will
encounter thermodynamics many different times during this year)
we are going to begin by covering thermochemistry. As an
introduction to thermochemistry I need to define several
important terms so that we might better understand the two
reactions we observed earlier. Those terms are energy,
temperature, heat and work.
Because energy is not tangible, as are material objects, it
become difficult to define it completely. The scientific
definition of energy is the capacity to do work or to transfer
heat. In the first reaction we saw, between aluminum and
iron(III) oxide energy was given off in the form of heat. No work
was done by the reaction, with the exception of pushing back the
atmosphere. So no useful work. There appears to have been energy
present in the reactants which is liberated when the products are
formed, as heat when the reactants were combined.
In the second reaction energy was absorbed when the reaction
occurred. This was evident when I touched the container. It felt
cold to the touch. Heat was transferred from my hand to the
flask. Again no useful work was done.
In either case we could have utilized the heat transfer to do
some work. When we discuss energy two forms of energy come to
mind potential energy and kinetic energy. Potential energy (U =
mgh: g = 9.8 m.sec-2) is energy stored in
an object by virtue of its position. A book held above my head
has more potential energy than a book held at my side near my
waist. The amount of potential energy an object has depends on
the mass of the object and its height above the earth's surface.
If I drop the book the potential energy is converted to kinetic
energy. At the atomic level two nitrogen atoms have a higher
potential energy when they are far apart then when they are
bonded together, just as the book has a higher potential when
separated from the surface of the earth. To separate the two
nitrogen atoms in a mole of N2 molecules requires 960
kJ. This amount of energy would raise a 100 g ball to a height of
600 miles.
Kinetic energy is energy of motion. The magnitude of the
kinetic energy of an object depends on its mass and velocity. Ek
= 1/2 mv2 The heavier and faster an object is
going the more energy it has and the more work it can do on
whatever.
If we substitute the SI units for mass and velocity into the
kinetic energy equation we have the correct units for energy. The
SI unit for energy is the joule (J) (1 kg.m2.s-2
). An object with a mass of 1 kg traveling at a velocity of 1 m.sec-1
has a kinetic energy of 1 J. One particular textbook author has
indicated that dropping a six pack of soda pop cans on your foot,
the kinetic energy at the moment of impact is between 4 and 10
joules. The kinetic energy of one gas molecule at 25 degree C is
about 6 x 10-21 J. A mole of this gas has about 4 kJ
of kinetic energy. If that energy could be harnessed it could be
used to raise 350 copies of our text about 1 meter.
A 100 watt light bulb produces 100 J of energy for every
second it operates. (When the City of Stillwater sends a bill for
the energy used at your house it includes an electrical bill for
energy used in the form of electricity and the units used are
kilowatt-hours. A single kilowatt-hour is equivalent to 1000
watts-3600 seconds or 3.6 x 106 watt-sec or 3.6 x 106
joules. A BTU is the amount of energy required to raise the
temperature of one pound of water one degree Fahrenheit.) A
calorie is another non-SI energy unit. It is defined as the heat
required to raise the temperature of 1 g of water, 1 degreeC. 1
cal = 4.184 J.
If we had had the proper equipment we could have measured the
temperature of both reactions. Temperature is a measure of the
degree of hotness or coldness of an object. If I indicate the
temperature of a sample of water is 95 C we know the sample is
very hot. If another sample of water has a temperature of 1C, we
know the sample will feel cool when we touch it.
Heat is energy that is transferred as a result of a
temperature difference. Heat always flows from a warmer object to
a cooler object. Heat causes a change in temperature. So when we
'heat' an object it get hotter. Lighting a bunsen burner and
placing it beneath a beaker filled with water causes the
temperature of the water to increase. Heat flows from the warmer
flame of the bunsen burner to the cooler water. We can measure this temperature change using
a thermometer. If we have two beakers, a 100 mL beaker and a
25 mL beaker each filled with water at the same initial
temperature, and an equal amount of heat is added to each beaker
we will find the water in the larger beaker to have a lower
temperature compared to the temperature of the water in the small
beaker. The temperature change depends on the amount of heat
added to the water and it depends on the amount of matter
present. When the bunsen burner is removed, the source of heat,
the water in the beaker will cool, as heat flows from the warmer
water in the beaker to the cooler air of the room, and return to
room temperature. I have described heat as flowing or being
transferred and this may be misleading. Heat is not matter, it is
not contained in matter. Heat is a way to exchange energy.
As we saw in the example of heating the two samples of water,
the addition of equal amounts of heat resulted in a different
change in temperature because of the different masses of water.
It is interesting that there is a relationship to the amount of
energy added to a specified amount of water and the subsequent
temperature change. This relationship is called specific heat.
Adding energy, in the form of heat, to a sample of water will
cause a change in temperature of the water. It is interesting
that if 4.184 J of energy is added to 1 gram of water at 4
degrees C the temperature of the sample changes by one degree
Celsius. This quantity of heat is called the specific heat of
water. This is the amount heat required to change the temperature
of 1 gram of water by 1 degree Celsius. The definition of
specific heat is;
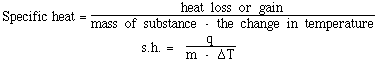
The specific heat of any substance can be determined using
the equation. All that must be measured is how much heat is
required to effect a change in temperature for a given amount of
the substance.
Some additional specific heats can be found in Table 5.2 on
page 159 of your textbook.
Water has a very high specific heat, that is, a large amount
of heat is required to change the temperature of water. The
greater the specific heat of a substance the more heat is
required to effect a change in temperature of 1 gram of the
substance.
Heat capacity is an alternative way to express the capacity
of a substance, or an object, to absorb heat. But heat capacity
has different units compared to specific heat. Heat capacity is
the amount of heat required to raise the temperature of an object
by 1 degree C. The difference between specific heat and heat
capacity is that specific heat is heat required per gram, while
heat capacity is heat required for an object whose mass is
constant and not expected to changed. This difference will be
important when we discuss calorimetry.
Once the specific heat or heat capacity of a
substance is known it is possible to determine the amount of heat
absorbed or lost in a process. The equation can be rearranged to
solve for heat. We will use the symbol, q, to represent heat.
Rearranging the equation, heat lose or gain = Specific heat
. mass of substance . the change in temperature
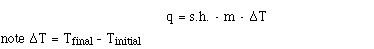
This equation tells us the quantity of heat but we also need
to know direction. If the sign of q is positive heat flows into
the system. If q is negative heat flows out of the system.
Lets look at some sample problems;
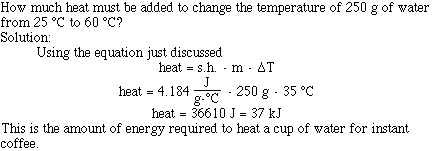
Problem #1;
Problem #2;
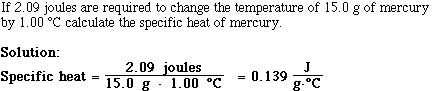
Problem #3;