 |
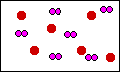
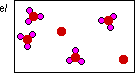
This diagram is correct. The total number of
sulfur and oxygen atoms are conserved in this diagram.
Notice there is an excess of sulfur atoms. The original
mixture did not have enough oxygen atoms to react with
all of hte sulfur. So dioxygen is the limiting reagent
and sulfur is in excess.
|
|