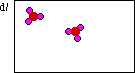 |
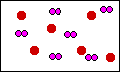
In this diagram the container has the correct
product, SO3. But the problem is there is not
enough product. The original diagram above shows 6
molecules of dioxygen, and six atoms of sulfur. This
digram shows only two molecules of SO3. There
is more product than is indicated here.
|
|