 |
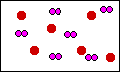
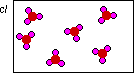
In this diagram at least the container has the
correct product, SO3. But the problem is there
is too much product. The original diagram above shows 6
molecules of dioxygen, for a total of twelve atoms. This
digram shows 18 atoms of oxygen. There are too many
oxygen atoms!
|
|